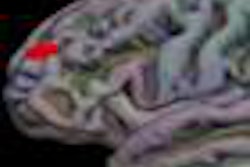
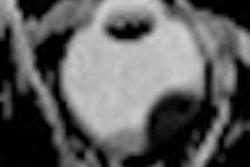
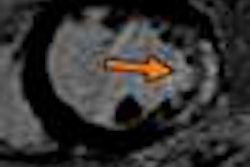
Medical device developer Monteris Medical has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its MRI-guided NeuroBlate laser ablation system.
The second-generation device uses a surgical laser to ablate diseased brain tissue and incorporates enhanced visualization capabilities through MRI.
The NeuroBlate system features neurosurgeon-controlled, 3D ablation through a software platform designed to support surgical decision-making during brain surgery and provide postprocedure confirmation of the effects of thermal therapy.