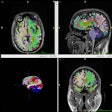
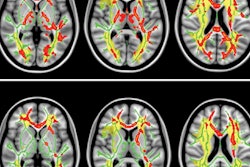
GE Healthcare has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its magnetic resonance image compilation (MAGIC) multicontrast MR technique.
GE collaborated with Swedish medical imaging software developer SyntheticMR to create MAGIC, which is designed to help clinicians modify image contrast after scanning has been completed. That option is not possible with conventional imaging, GE said.
The acquisition technique also provides more data than conventional scanning in less time, according to GE.
MAGIC is available on GE's Optima MR450, Optima MR450w, Discovery MR750, Discovery MR750w, and Signa Pioneer scanners.


.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)




.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)