Radiologists at Emory University are getting by with a little help from their electrophysiology friends. Together they are successfully ensuring the safety of patients with cardiac implantable electronic devices (CIEDs) during MRI scans, according to a paper published in the September issue of the American Journal of Roentgenology.
By having electrophysiologists screen and, if necessary, reprogram implantable devices before patients enter the MRI suite, diagnostic image quality is achieved, artifacts are reduced to a minimum, and workflow is enhanced. And Emory radiologists are quick to give credit where credit is due (AJR, Vol. 207:3, pp. 599-604).
"For radiologists who are thinking of developing a workflow like this, a critically important component is the electrophysiologists," said corresponding author Dr. Courtney Coursey Moreno, an associate professor of radiology at Emory. "They are the individuals who really do the vast majority of screening on the front end to make sure patients are safe to enter the MRI environment."
Device implants
It is estimated that some 400,000 people receive pacemakers and implantable cardioverter defibrillators (ICDs) each year in the U.S. That figure adds to the more than 3 million Americans already living with implanted cardiac devices.
 Dr. Courtney Coursey Moreno from Emory University.
Dr. Courtney Coursey Moreno from Emory University.Naturally, there are safety concerns about how implanted devices will react when exposed to the magnetic field of MRI scanners. There have been reports of devices heating, malfunctioning, and even moving due to magnetic forces.
The U.S. Food and Drug Administration (FDA) currently has two categories of CIEDs: conventional and MRI-conditional devices. The FDA considers conventional devices to be contraindicated for MRI scanning because the magnetic field can cause devices to malfunction, the authors noted.
In 2011, the agency approved MRI-conditional implantable devices after manufacturers modified leads and made other engineering changes. However, many of these MRI-conditional devices must be reprogrammed before and after the scans.
Because of the relative novelty of MRI scanning of implantable devices, the Emory researchers decided to examine both the safety of scanning and its effect on workflow.
"We decided to formally evaluate the safety of the workflow and the diagnostic quality of the images that were produced as a result," Moreno said.
The Emory process
Emory's protocol begins when the radiologist receives a request for an MRI scan of a patient with a cardiac device. Once he or she determines the scan is clinically appropriate, an electrophysiologist is brought in to consult with the patient, evaluate the kind of CIED implanted, and see if the device needs to be reprogrammed prior to MR imaging.
Most important is Emory's list for patients with a CIED who cannot be scanned, which includes the following:
- Age younger than 18 years
- Weight less than 80 lbs
- Being in the first trimester of pregnancy
- Having a pacemaker manufactured before 1996 or ICD manufactured before 2000
- Having a device implanted within the preceding three months
- Having abandoned or capped leads or leads implanted within the preceding four weeks
- Having active coronary ischemia, myocardial infarction, or cardiothoracic surgery within the previous three months
"Folks with an MRI-conditional device can't just walk in and have an MRI scan," Moreno told AuntMinnie.com. "They have to go through an evaluation by an electrophysiologist, and their devices still have to be adjusted before and after the scan."
When the standards are satisfied, an MRI technologist schedules the scan with the electrophysiologist. Both are present for the procedure. When the scan is complete, the electrophysiologist reprograms the implantable device back to its original settings.
"At Emory, we do add approximately 30 minutes to MRI appointment times when a pacemaker is involved," Moreno said. "We also found that it works best when one of our MRI technologists takes the lead and coordinates scheduling with the electrophysiologist for the [prescan] interrogation."
Diagnostic quality
To evaluate their protocol, the researchers studied 68 men and 36 women (mean age, 66 years; range: 20-89 years) with cardiac implantable devices who underwent a total of 113 scans on 1.5-tesla MRI systems. Seventy-four subjects had pacemakers and 39 had ICDs. Among the individuals with pacemakers, 60 (81%) had conventional devices and 14 (19%) had MRI-conditional devices. Device reprogramming was required for 71 (63%) MRI studies.
There were 42 different models of implantable devices in the study, all made by the five U.S. manufacturers: Biotronik, Boston Scientific, ELA Medical, Medtronic, and St. Jude Medical.
In the end, all MR images were of diagnostic quality for the targeted clinical application. Three studies (4%) had artifacts in the chest wall, which were due to the presence of the device's pulse generator in the field-of-view, and limited evaluation of tissue adjacent to the device. All other MRI scans were artifact-free.
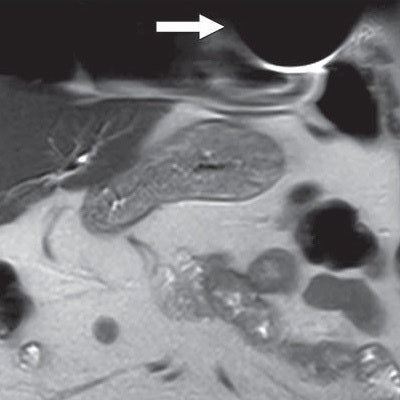
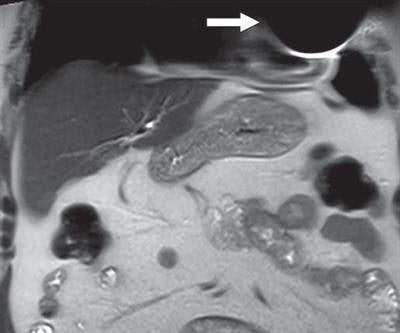 Coronal T2-weighted image shows susceptibility artifact related to cardiac implantable electronic device (arrow), which limited evaluation of portions of left anterior chest wall, lingula, and heart. Image courtesy of AJR.
Coronal T2-weighted image shows susceptibility artifact related to cardiac implantable electronic device (arrow), which limited evaluation of portions of left anterior chest wall, lingula, and heart. Image courtesy of AJR.Three patients (4%) reported minor adverse symptoms of heating or "tingling" at the pocket site and palpitations. The side effects were not severe enough to cancel the exam.
"If you are a radiologist and you want to establish this workflow at your institution, you really have to engage electrophysiologists," Moreno emphasized. "They are the individuals who really do the vast majority of screening on the front end to make sure patients are safe to enter the MRI environment. They are a huge part of this workflow."
AHA recommendations
Moreno also recommended that radiologists review guidelines from the American Heart Association (AHA) on how to perform MRI scans on patients with conventional and MRI-conditional devices.
"The document may be more in the electrophysiologist's area of expertise, but [the protocol] is now more mainstream in many imaging centers," she said.
Radiologists should also be aware that Medicare does not currently reimburse for MRI scans of patients with conventional implantable devices.
"We hope to conduct a larger retrospective study to analyze safety in a larger cohort of patients," she said. "We are hoping that Medicare will reconsider its decision so that individuals with conventional devices can have their MRI scans covered."