If imaging centers adhere to standard MRI parameters for static, gradient, and radiofrequency magnetic fields, implanted cardiac devices (ICDs) will continue to function smoothly during an MRI scan, according to a study published March 3 in Radiology.
Researchers monitored specific absorption rate, energy dose, and changes in magnetic field strength from more than 2,000 MRI exams and observed no significant adverse effects on ICD or lead performance related to the scanner's operation.
"These findings are particularly relevant for patients with implanted legacy devices that are not considered to be MRI conditional, as the need to scan them will continue for many years," wrote the authors, led by Dr. Amir Ali Rahsepar from Johns Hopkins University School of Medicine. "On the basis of our results, the risk of subsequent device malfunction or failure is low, if an MRI safety protocol ... is followed."
Even with technological advances in MRI scanners over the decades, concerns remain about how implanted cardiac devices such as pacemakers, implantable cardioverter defibrillators, and cardiac resynchronization therapy systems will perform when patients are inside the magnet bore.
The amplitude of the static and gradient magnetic fields of a 1.5-tesla scanner, for example, "are switched rapidly," the authors explained. Those changes can create conditions that potentially disrupt normal ICD performance. In addition, the radiofrequency fields of MRI scanners have been known to heat tissue adjacent to pacemakers and defibrillator leads.
To date, however, scant research exists on the association between changes in magnetic and radiofrequency fields during MRI scans and how those variations might affect implanted cardiac devices.
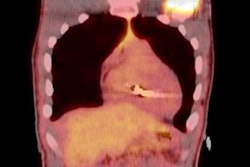
To determine if there is a connection, Rahsepar and colleagues prospectively analyzed 2,028 MRI scans between October 2003 and January 2015 from 1,464 patients (mean age, 67 ± 15 years) with implanted cardiac devices. Among the participants, 853 people (58%) had a permanent pacemaker, 454 individuals (31%) had an implantable cardioverter defibrillator, and 157 patients (11%) had a cardiac resynchronization therapy defibrillator. Those devices included a total of 2,755 leads. All of the MRI scans were performed on 1.5-tesla systems (Avanto or Aera, Siemens Healthineers) using routine protocols with standard whole-body specific absorption rate settings.
The researchers then calculated whole-body values for specific absorption rate, changes in magnetic field per unit time, imaging region, and scan time for each MRI pulse sequence and compared those results with device function.
Most importantly, the researchers found no adverse associations between the MRI scanner's whole body-averaged specific absorption rates, specific energy doses, gradient field slew rates, scan durations, and imaging regions and any changes in ICD performance or lead parameters. One minor negative reaction was reported: Thoracic MRI scans were associated with decreased battery voltage within 10 minutes after the exam.
The encouraging results were further endorsed in an accompanying editorial by Frank Shellock, PhD, an adjunct professor of clinical physical therapy at University of Southern California.
"This well-conducted investigation by Rahsepar et al greatly contributes to the ever-growing body of peer-reviewed literature that substantiates the claim that patients with non-MR-conditional CIEDs may safely undergo MRI, provided specific safety guidelines are followed," Shellock wrote.