German researchers have found additional evidence that the use of a macrocyclic gadolinium-based contrast agent (GBCA) for MRI scans does not increase signal intensity in the dentate nucleus. The new findings come from a study of pediatric patients published online in Radiology.
The results mirror findings from a previous study in which the researchers detected no evidence of increased T1 signal intensity among adult patients who received more than 20 injections of macrocyclic GBCAs for their MRI scans. In this case, pediatric patients received a mean of 8.6 serial injections of a macrocyclic GBCA and exhibited no heightened signal intensity after their scans (Radiology, March 8, 2017).
"We have shown in multiple studies in adults that we don't see any deposition with the macrocyclic gadolinium-based contrast agents. Actually, that is exactly what we expected in children," said lead author Dr. Alexander Radbruch, senior attending physician at the German Cancer Research Center in Heidelberg. "There is no physiological reason why you should see a difference between children and adults. The physiology is the same and the chemistry does not change."
Mounting evidence
The current study follows two recently published papers by Radbruch et al on macrocyclic GBCAs within an adult patient population. In November 2016, the authors reported that switching from a linear GBCA to a macrocyclic GBCA reduced the level of residual signal intensity in the brains of patients who underwent multiple enhanced MRI scans with both types of agents.
 Dr. Alexander Radbruch from Heidelberg University.
Dr. Alexander Radbruch from Heidelberg University.One month later, the researchers published a review of 33 adult patients who received a mean of 23 serial injections of macrocyclic GBCAs, with no significant T1 signal intensity appearing in the dentate nucleus.
In the current study, Radbruch led a team that examined 41 pediatric patients ranging in age from 3 to 17 years who underwent at least five consecutive MRI scans on the same 1.5-tesla system (Magnetom Avanto, Siemens Healthineers) with a head matrix coil. They also all received the same gadoterate meglumine macrocyclic GBCA (Dotarem, Guerbet).
The mean number of intravenous injections per pediatric patient was 8.6 ± 3.9, with a mean injected dose of gadoterate meglumine per MRI exam of 3.72 mmol, or 0.1 mmol per kilogram based on the patient's body weight. The average time between each administration was 16.7 ± 7.9 weeks.
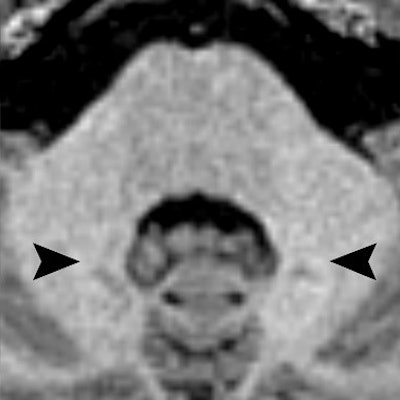
As in the previous research in adults, Radbruch and colleagues focused on signal intensity in the dentate nucleus on unenhanced T1-weighted MR images. Signal intensity ratio differences were calculated based on dentate nucleus-to-pons and dentate nucleus-to-middle cerebellar peduncle values by subtracting the signal intensity ratio of the first MRI scan from the signal intensity ratio of the last MRI exam.
The ratio differences did not vary significantly from 0, meaning there was no increase in T1 signal intensity. The dentate nucleus-to-pons ratio difference between the first and last MR scans was -0.0012 ± 0.0101 (p = 0.4360), while the dentate nucleus-to-middle cerebellar peduncle ratio difference was 0.0007 ± 0.0088 (p = 0.604).
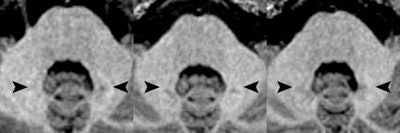 Axial MR images of a patient before (left) and after four (middle) and eight (right) administrations of the macrocyclic GBCA gadoterate meglumine. Location of the hyperintense dentate nucleus (arrowheads) is evident, with the patient's pre-existing hyperintensities due to 14 prior administrations of linear GBCAs. Images courtesy of Radiology.
Axial MR images of a patient before (left) and after four (middle) and eight (right) administrations of the macrocyclic GBCA gadoterate meglumine. Location of the hyperintense dentate nucleus (arrowheads) is evident, with the patient's pre-existing hyperintensities due to 14 prior administrations of linear GBCAs. Images courtesy of Radiology.Study strengths
The findings are particularly noteworthy for pediatric patients, since some could undergo multiple GBCA-enhanced MRI scans during their lives.
"I think it is obvious that these children who might have to live with [gadolinium] deposition for their whole lives are extremely vulnerable patients," Radbruch said. "We owe this to our patients to clarify the issue and to have a look if there are differences between the gadolinium-based contrast agents for children."
The strength of this study, the authors noted, is that all of the subjects were assessed with identical imaging parameters using the same 1.5-tesla scanner. Varying imaging parameters can significantly affect signal intensity in the dentate nucleus and could cloud the results.
"Often no conclusions can be drawn from these data," Radbruch said. "We need to have standards for the future."
As such, Radbruch is a member of the Gadolinium Retention Evaluation Consortium, which is in the process of establishing standards for retrospective patient studies and collecting clinical experiences and outcomes with gadolinium contrast agents to store in a database for fellow researchers.
Radbruch and colleagues cautioned that they could not discount the possibility that larger amounts of gadoterate meglumine eventually might result in increased signal intensity in the dentate nucleus.
"However, it should be noted that even in one patient with 23 serial injections of gadoterate meglumine, no signal intensity increase was found," the authors wrote.
As for the GBCA protocol at the University of Heidelberg Medical Center, clinicians exclusively use macrocyclic contrast agents, except for liver imaging when they use a linear GBCA for more specialized results.
"Actually, we have used the same protocol prior to recent studies, so we do not have to change our way of treating patients," Radbruch said. "We always have been cautious, so this whole debate has not changed our way of using contrast."


.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)





.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)











