Dutch researchers are reporting promising results using a new urinary biomarker risk-scoring system to test for the possibility of prostate cancer. The test could be used to select which patients should be sent on for additional MRI scans or biopsy, according to a study published in the October issue of The Prostate.
The researchers compared the SelectMDx score with findings from multiparametric MRI (mpMRI) exams to determine how well the biomarker score works in detecting individuals at risk for prostate cancer. They found that the exams correlated well, raising the possibility that SelectMDx could be used to triage patients for additional workup.
"Our results suggest that this risk score could guide clinicians in identifying patients at risk for significant prostate cancer and selecting patients for further radiological diagnostics to reduce unnecessary procedures," wrote lead author Dr. Rianne Hendriks and colleagues from Radboud University Medical Center in Nijmegen, the Netherlands (Prostate, October 2017, Vol. 77:14, pp. 1401-1407).
Subpar diagnoses
Diagnoses of prostate cancer are primarily accomplished through digital rectal exams (DREs), serum prostate-specific antigen (PSA) tests, and transrectal ultrasound (TRUS)-guided biopsy. One problem is that PSA tests often fall short on specificity for prostate cancer, which can lead to unnecessary biopsies and overdiagnoses of low-risk localized disease.
 Co-author Dr. Peter Mulders, chairman of the urology department at Radboud University.
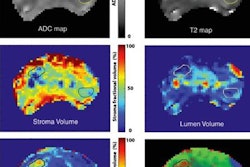
Co-author Dr. Peter Mulders, chairman of the urology department at Radboud University.More recently, MRI has gained favor with clinicians who have found the modality to enhance prostate cancer detection and tumor localization through multiparametric imaging techniques that have high sensitivity and high negative predictive value. A typical multiparametric protocol combines T2-weighted anatomical sequences, diffusion-weighted imaging (DWI), dynamic contrast-enhanced (DCE) MRI, and MR spectroscopy.
At the same time, new biomarkers have been developed to better predict which patients should receive further workup or biopsy, as reflected in a Gleason score of at least 6.
"It has been shown that mpMRI in expert hands is superior in visualizing lesions in the prostate with Gleason score 4 and 5 prostate cancer when compared to ultrasound-guided core biopsy procedures," wrote study co-authors Drs. Peter Mulders and Jack Schalken in an email to AuntMinnie.com. "We sought to develop a noninvasive test that predicted the presence of clinically significant prostate cancer."
SelectMDx approach
The SelectMDx method, developed by MDxHealth in Irvine, CA, starts by measuring gene expression levels from the HOXC6 and DLX1 coding genes, collected from urine after a digital rectal exam. It combines this with clinical risk factors such as the patient's age, prostate-specific antigen (PSA) levels, PSA density, family history, and prior negative prostate biopsies.
 Co-author Dr. Jack Schalken, research director of urology at Radboud.
Co-author Dr. Jack Schalken, research director of urology at Radboud.For the study, the researchers retrospectively analyzed 172 patients with a mean age of 63 years (± 6.3 years) and a median PSA level of 7.4 (range, 5.3-11.7). Subjects also had undergone one or more prostate biopsies based on elevated PSA levels, an abnormal DRE, or a family history of prostate cancer.
Of those patients, 100 (58%) were diagnosed with prostate cancer based on biopsy, with 52 (52%) having high-grade prostate cancer (Gleason score ≥ 7). Multiparametric MRI scans were ordered based on persistent clinical suspicion of prostate cancer after negative prostate biopsies or prostate cancer staging after positive biopsies.
The mpMRI exams were performed on a 3-tesla scanner (Magnetom Trio or Skyra, Siemens Healthineers) at the medical center between November 2009 and March 2016. The protocol included T2-weighted MRI, DWI-MRI, and DCE-MRI.
TRUS-guided prostate biopsies were performed, with histological grading based on the Gleason system. Urine samples were collected after the digital rectal exam and prior to biopsy to measure expression levels of the coding genes HOXC6, DLX1, and KLK3. Those results were then combined with clinical prostate cancer risk factors, such as age, DRE results, PSA, PSA density, family history, and prior negative prostate biopsies, to determine the SelectMDx score.
Based on previous research, the chosen cutoff point for the SelectMDx score was -2.8 to achieve a sensitivity of 96% and a negative predictive value of 98% for high-grade prostate cancer.
The final scores
SelectMDx scores had a strong association with mpMRI results in predicting patients who were considered suspicious for significant prostate cancer, based on a PI-RADS score of 4-5, the researchers found. SelectMDx also outperformed PSA and prostate cancer antigen 3 (PCA3) tests, with an area under the curve (AUC) of 0.83 versus 0.66 and 0.65, respectively.
 Jan Groen, PhD, MDxHealth CEO
Jan Groen, PhD, MDxHealth CEO"Based on the high negative predictive value for clinically significant prostate cancer, we believe [SelectMDx] is safe," Mulders and Schalken said. "However, it remains a shared decision by the urologist and the patient."
Currently, the SelectMDx test is available in the U.S. and Europe. MDxHealth also offers an in vitro diagnostic kit to hospitals in Europe, which allows the facilities to run the test in house. Outside Europe, MDxHealth has signed several distribution agreements to make SelectMDx available in Asia and the Middle East.
SelectMDx is not available directly to imaging facilities, because the test requires a urine sample that needs to be run in a molecular diagnostic laboratory, wrote Jan Groen, PhD, MDxHealth's CEO, in an email to AuntMinnie.com. But the company believes that use of the test will help select who should receive additional workup.
"The result of this test will ensure radiologists are referred the men most likely to have clinically significant cancer," Groen said.