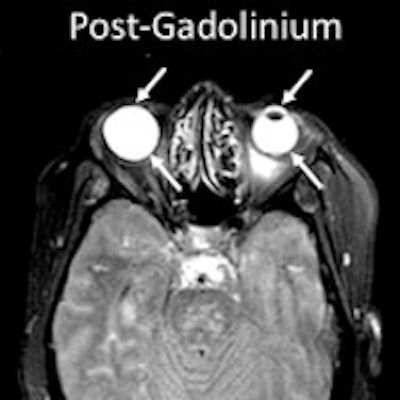
Researchers from the U.S. National Institutes of Health (NIH) found that gadolinium from MRI contrast can leak into the eyes of stroke patients who receive scans. They don't understand the mechanism of the leakage, although it may have some clinical consequences, according to a February 7 paper in Neurology.
The gadolinium was administered on initial MRI scans when the stroke patients first came to the hospital and again on two-hour and 24-hour follow-up imaging exams. As for the clinical significance of the phenomenon, patients with gadolinium leakage in both eye chambers at two hours had larger brain infarcts.
"Probably the biggest finding in this study is that there is a process going on in the brain that is having an effect on the process of gadolinium leaking into the eye," said senior author Dr. Richard Leigh, an assistant clinical investigator at the NIH's National Institute of Neurological Disorders and Stroke (NINDS). "That suggests that whatever is happening in the brain is affecting the eye, or alternately there is some sort of diffuse process that is affecting both the brain and the eye."
An unexpected finding
The researchers were prompted to explore the presence of gadolinium in the eyes through a rather unexpected finding. When a patient enters the hospital for a possible stroke, it is almost automatic that he or she undergoes an MRI brain scan, often with a gadolinium-based contrast agent.
In the case of a stroke, the blood-brain barrier is disrupted, allowing the gadolinium to enter the brain parenchyma or cerebrospinal fluid (CSF). In the absence of gadolinium, the CSF is suppressed and typically dark on MRI scans in which a fluid-attenuated inversion recovery (FLAIR) protocol is used. When gadolinium is present, it changes the CSF's nuclear MR properties and the fluid becomes illuminated. Naturally, during the MRI scan of the brain, the eyes are included in some images.
"The fluids in the eye are similar in terms of their properties to CSF, so they also are suppressed on the FLAIR scan," Leigh explained. "We started to notice that when gadolinium would leak into the brain, it would also sometimes leak into the eye, causing the fluid in the eyes not to suppress; the eyes would become bright. We weren't quite sure what to make of it, and that's why we embarked on this study."
Leigh and colleagues included 167 stroke patients who were given gadolinium with their initial MRI scan upon admission to the hospital to determine the extent of stroke. The subjects were enrolled in the NIH's Natural History of Stroke study in 2013 and 2014.
The patients had the initial MRI scan for a baseline reading and follow-up MRI FLAIR scans approximately two and 24 hours later. The exams were conducted on 1.5-tesla (Signa, GE Healthcare) and 3-tesla scanners (Skyra, Siemens Healthineers; Achieva, Philips Healthcare).
Among the subjects, 109 (65%) were treated with IV tissue plasminogen activator (tPA), while 58 (35%) did not receive tPA. Previous studies have indicated that tPA can affect the blood-brain barrier, so the researchers were curious to see how this stroke treatment might influence the presence of gadolinium in the eyes.
The eyes have it
The researchers observed gadolinium leakage in 127 patients (76%) in either the aqueous or vitreous chamber of the eye on at least one of the follow-up MRI scans.
At the two-hour follow-up, gadolinium leakage was detected in 51 patients (64%); it was more common in the aqueous chamber (34 patients, 67%) than the vitreous chamber (3 patients, 6%). Interestingly, the 14 patients with gadolinium leakage in both eye chambers at two hours had larger diffusion lesion volume or infarcts (28.34 mL) when compared to patients without this pattern of gadolinium leakage (8.16 mL) (p = 0.022).
At the 24-hour follow-up scan, gadolinium leakage was much more common, appearing in a total of 121 patients (75%). All 121 cases of leakage appeared in the vitreous chamber. In 10 patients (8%), gadolinium was evident in both eye chambers.
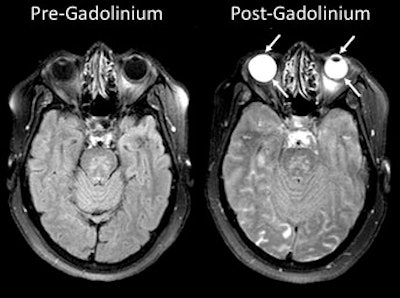 MRI scans show gadolinium leakage in the eyes after the contrast agent is administered to a stroke patient. Images courtesy of the NINDS.
MRI scans show gadolinium leakage in the eyes after the contrast agent is administered to a stroke patient. Images courtesy of the NINDS.Regarding the influence of tPA, gadolinium was present in 86 patients (79%) who received it and in 41 patients (71%) who did not. There was no statistically significant association between the administration of tPA and the occurrence of gadolinium leakage (p = 0.695).
"We found no suggestion of an effect where tPA either increased or decreased the amount of gadolinium leakage in the eye," Leigh said. "It is hard to say for sure that it is not involved, because not identifying an association does not mean that one does not exist. But there was certainly no suggestion that there was any influence of tPA on this process."
More questions
While the findings are certainly eye-opening, Leigh said the results raise more questions than provide answers.
"We established that using this imaging biomarker, we are finding that there is a relationship between what is happening in the eye and what is happening in the brain," he said. "We don't have enough information to say that one is directly causal to the other. Because of that, we don't have any specific changes in stroke management or evaluation currently as a result of this study."
And how does the gadolinium travel from the brain to the eye? Well, it's too soon to determine that answer as well. One possible route is through disruption of the blood-ocular barrier. Another possibility is the eye's connection to cerebrospinal fluid through the optic nerve.
"It is possible that [gadolinium] could be coming from either source," Leigh said. "We cannot say definitively which is the case in our study. However, my feeling is that it is most likely coming through the blood, and when we are seeing their disruption of this blood-ocular barrier, that's where we are seeing leakage of the gadolinium to the fluids of the eye."
In addition, gadolinium leakage into the vitreous chamber appeared to correlate well with changes in the brain that are associated with vascular dementia and vascular cognitive impairment. It begs the question of whether clinicians can use the condition of the eye to assess a person's cognitive status or risk of developing cognitive decline and other vascular disease.
Regarding the ongoing debate about any adverse effects from gadolinium retention in the brain and other parts of the body, this study confirmed that the element moves through various regions, as well as in the blood. The good news from the preliminary results is that patients experienced no visual problems or any other ocular issues as a result of the gadolinium leakage.
"We checked to make sure, and there did not appear to be any direct effect of the gadolinium itself on the visual systems, based on the data we had," Leigh said. "I would say this data adds to the body of literature that gadolinium enters the two chambers, but we were not able to identify any negative impact of this."