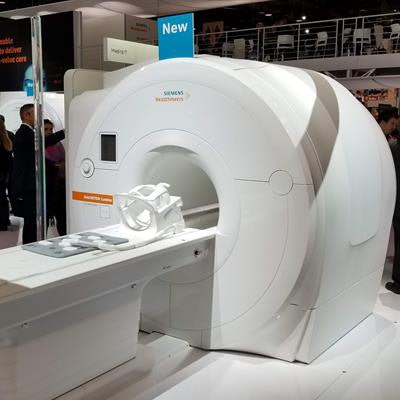
Siemens Healthineers has received clearance from the U.S. Food and Drug Administration (FDA) for its 3-tesla Magnetom Lumina MRI scanner.
Siemens launched the scanner at RSNA 2018, and it features the company's BioMatrix patient personalization technology, a 70-cm bore, and the company's Go technologies powered by artificial intelligence (AI) to accelerate workflow.
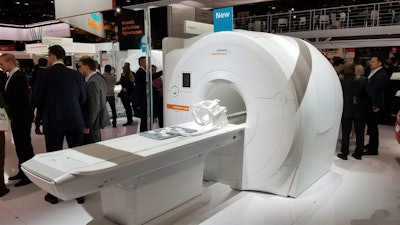 Siemens' Magnetom Lumina 3-tesla scanner.
Siemens' Magnetom Lumina 3-tesla scanner.Magnetom Lumina's new Turbo Suite acceleration packages are designed to reduce scan times for routine musculoskeletal exams of various parts of the body by as much as 50%, according to the company.
Siemens also offers an optional dockable table for Magnetom Lumina and plans to offer an optional Innovision in-bore Infotainment system. The technology would give patients the illusion of being inside a larger bore and provide video and sound to ease anxiety during the scan. Innovision is currently under development.




















