A prototype low-field MRI scanner operating at 0.55 tesla had advantages compared with 1.5 tesla for interventional MRI-guided procedures, particularly for patients with implanted devices, according to a study published online October 1 in Radiology.
Researchers from the U.S. National Institutes of Health (NIH) and Siemens Healthineers modified a commercial 1.5-tesla scanner (Magnetom Aera, Siemens) to operate at a 0.55-tesla field strength. They then tested the new system to perform a number of scans, including MRI-guided cardiovascular catheterizations. The results were particularly satisfying for patients who experienced no heating of their implanted cardiac devices, which are considered unsafe at 1.5 tesla.
"The lack of safe metal devices has limited MRI guidance of cardiovascular procedures," wrote the authors, led by Adrienne Campbell-Washburn, PhD, from the NIH's National Heart, Lung, and Blood Institute. "We anticipate that low-field strength MRI will enable MRI-guided procedures that use a subset of devices from the traditional catheterization environment."
The recent trend in medical imaging has been to increase magnet strength to 3 tesla and 7 tesla for improved signal-to-noise ratio (SNR) and finer image resolution. As a result, manufacturers have shown less interest in developing lower field systems, which "have been largely overlooked as hardware and software have improved over the last two decades and, therefore, are not well-suited for technically demanding imaging," the authors wrote.
There can be, however, a downside to greater magnet strength, with image distortion, reduced imaging efficiency, and higher equipment costs. Conversely, low field strength might offer advantages for some clinical applications.
For example, short T1- and long T2-weighted imaging allow for more efficient pulse sequence design, and imaging of air-tissue interfaces can be improved through reduced susceptibility gradients, the authors noted. Hence, the researchers sought to develop a customized 0.55-tesla MRI scanner equipped with a contemporary magnet, gradient, and radiofrequency (RF) components for advanced imaging applications.
A total of 83 0.55-tesla MRI scans were performed on 83 subjects (mean age, 34 ± 13 years). Depending on the scan, they were fitted with phased-array receiver coils, such as 18-channel spine array, six-channel body array, and/or a 16-channel head array. Subjects also underwent 1.5-tesla scans for comparison MR images.
Among the devices implanted in the subjects were 16 commercial nitinol guidewires and stainless steel braided catheters, considered unsafe due to the possibility of the implants heating during MRI-guided catheterization at 1.5 tesla. For this exercise, the researchers set a safety margin of less than 1° C of heating at the device tip during two minutes of real-time, continuous imaging at a high specific absorption rate.
The NIH-Siemens system performed quite well, with nine metallic interventional devices found to be safe with increased heating of less than 1° C and MRI-guided right heart catheterization successfully performed and completed in seven study participants with commercial metallic guidewires.
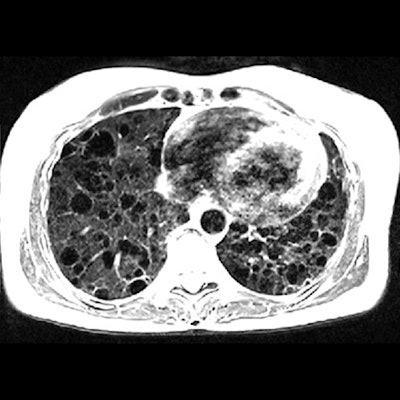
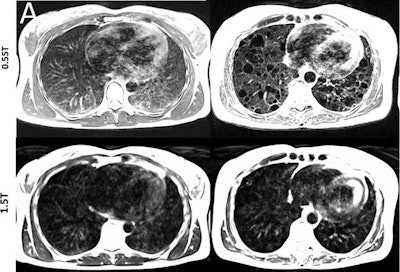 Lung images show increased signal intensity at 0.55 tesla, compared with 1.5 tesla, because of improved field homogeneity, as seen in a healthy 26-year-old woman and a 54-year-old woman with a rare lung disease known as lymphangioleiomyomatosis. Images courtesy of Radiology.
Lung images show increased signal intensity at 0.55 tesla, compared with 1.5 tesla, because of improved field homogeneity, as seen in a healthy 26-year-old woman and a 54-year-old woman with a rare lung disease known as lymphangioleiomyomatosis. Images courtesy of Radiology.In addition, compared with 1.5 tesla, image distortion was reduced at 0.55 tesla in scans of the lungs, upper airway, cranial sinuses, and intestines due to improved field homogeneity. In particular, oxygen inhalation generated lung signal enhancement of 19% (± 11) at 0.55 tesla, compared with 7.6% (± 6.3) at 1.5 tesla.
"By using a high-performance low field strength MRI system, there are opportunities for the development of new techniques for invasive MRI-guided procedures, to design acquisition and reconstruction strategies, and for contrast agent development and hardware design," the researchers concluded. "This technology warrants further investigation in the context of clinical MRI."