Could a manganese-based MRI contrast agent (MBCA) provide equivalent MR image quality and help alleviate concerns surrounding the use of gadolinium-based contrast agents (GBCAs)? New research indicates that it has some potential.
A group of researchers from Massachusetts General Hospital (MGH) in Boston is collaborating with Reveal Pharmaceuticals of Cambridge, MA, to develop Mn-PyC3A, which thus far has provided comparable or enhanced image quality in preclinical comparisons with several GBCAs and has shown that it can be secreted from the body significantly more quickly and efficiently than gadolinium.
"From talking to my radiology colleagues, if we can give them an effective gadolinium-free replacement that requires no additional learning curve, then that would be the ideal product for them," said Peter Caravan, PhD, co-developer and co-director at the Institute for Innovation in Imaging at MGH.
MBCA development
The researchers' interest in developing an MRI contrast agent that's not based on gadolinium began several years ago following studies that connected the administration of GBCAs to patients with poor renal function to their eventual progression to nephrogenic systemic fibrosis (NSF). While the frequency of NSF cases dwindled to near almost none in the last decade with the implementation of new safety guidelines, a new problem has arisen with the discovery that trace elements of gadolinium are remaining in many patients years after their scans.
Manganese-based MR contrast agents are not new to MR imaging, but the challenge with manganese is that it does not always stay in chelate form to create a signal, or image, from the MRI scan. As opposed to gadolinium, which is a toxic element, manganese in small amounts is an essential element already in the human body and is even sold as a dietary supplement.
"Even if there were a little bit of manganese that was released or stayed in the body, as long as that amount was quite small, it would not be a problem," Caravan explained. "It would either be incorporated into your body's pool of manganese that is needed to survive or be eliminated. The body has good mechanisms for regulating how much manganese it needs."
The researchers believe, at this point, they have overcome the issues of chelation and the manganese that is injected, at least in animal models, is retained at much lower levels than the gadolinium chelate in GBCAs and is secreted more efficiently than any other element, and any manganese left over in the body is benign.
"Gadolinium has no function in biology. Any gadolinium present in your body should not be there," Caravan added. "So I think that is another element of safety that this [manganese] approach will bring."
Preclinical results
In their most recent study, the researchers separately compared the efficacy and image quality of Mn-PyC3A against gadoteric acid (Dotarem, Guerbet) to detect breast cancer tumors and against gadoxetate disodium (Eovist, Bayer HealthCare) to identify metastatic liver disease in rodent models (Investigative Radiology, November 2019, Vol. 54:11, pp. 697-703). The second objective was to see how well Mn-PyC3A and the two GBCAs were excreted from the rodents' bodies over the course of seven days.
In evaluating the results, the researchers found that Mn-PyC3A achieved greater contrast-to-noise ratios in comparisons with both GBCAs to provide sufficiently adequate image quality for both the breast and liver. In addition, after one day, the percentage of injected dose of manganese still remaining in the rodents' systems (0.32 ± 0.2) was significantly less than that of gadolinium (0.57 ± 0.12) (p = 0.003). After seven days, the percentage of leftover manganese (0.058 ± 0.051) in the rodents again was significantly less than the percentage of residual gadolinium (0.19 ± 0.052) (p < 0.0001).
Caravan and his colleagues also reported similar success in a November 2017 study that concluded that the MBCA was equivalent to gadolinium-based contrast for enhancement of a baboon's blood vessels. Mn-PyC3A also was quickly excreted through both kidney and liver clearance, leaving no evidence of the release of "free manganese" in these subjects.
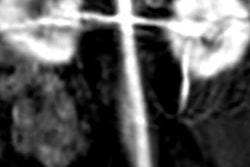
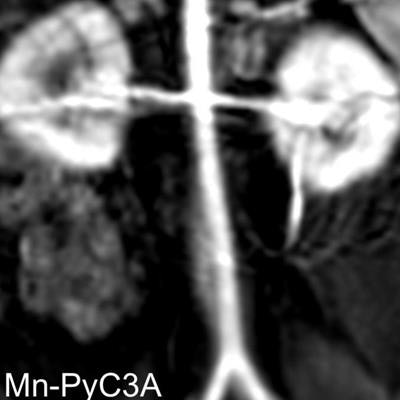
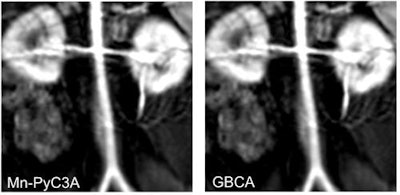 Contrast-enhanced MR image of the abdominal aorta, renal arteries, and kidneys of a baboon acquired with the manganese-based agent Mn-PyC3A (left), compared with the same scan with a gadolinium-based contrast agent (right). Images courtesy of Caravan et al.
Contrast-enhanced MR image of the abdominal aorta, renal arteries, and kidneys of a baboon acquired with the manganese-based agent Mn-PyC3A (left), compared with the same scan with a gadolinium-based contrast agent (right). Images courtesy of Caravan et al.Perhaps most importantly, the researchers have seen no adverse side effects from the MBCA in preclinical studies.
"We have shown repeatedly that we get equivalent contrast," Caravan said. "In well-designed head-to-head studies, there is really no concern."
As they were to extrapolate these results to human patients, the goals remain making Mn-PyC3A the functional equivalent of gadolinium in regard to both quality of MR images and in terms of patient workflow.
"The radiologist would have the same vial, the same volume of a colorless liquid would be injected at the same rate, and the same scanning protocols. Nothing would change. We believe we have achieved that," Caravan said.
GBCA concerns
Like many radiological researchers, Caravan has been following the debate over the potential long-term health effects of lingering traces of gadolinium in brain tissue, the liver, and bone years after administration of contrast for an MRI scan.
"Although the community is very focused on the brain, it is a little like looking for your keys under the lamppost," he added. "We can see signal intensity in the brain, but from autopsy and biopsy data, we know that the concentration and amount of gadolinium and other organs is much higher. So, if we can give the same information without the need to use a toxic heavy metal, then why not do it?"
As for the progression to human clinical trials, the researchers are currently in the process of fundraising to move their MBCA forward. The researchers have been in discussion with a number of contrast media companies and venture capitalists. Along with Reveal Pharmaceuticals, they also have raised money from private investors and received grants from the U.S. National Institute for Biomedical Imaging and Bioengineering, National Heart Lung and Blood Institute, and the National Institute of Diabetes and Digestive and Kidney Diseases.
"To put this in perspective, contrast agents are drugs and are regulated as such," Caravan added. "So the drug development process is quite expensive and a bit time-consuming. It does take a significant investment to get into clinical trials."
Thus far, the financial hurdles have not deterred Caravan and his colleagues from their mission.