MRI-guided focused ultrasound (MRgFUS) shows promise as an alternative to surgery or radiation therapy in patients with intermediate-risk prostate cancer, according to a study published February 2 in Radiology.
The results mean that a significant number of patients could receive prostate cancer treatment that doesn't negatively affect their quality of life, according to a statement released by the RSNA: About 20% to 30% of prostate cancer patients who go on to get surgery or radiation therapy would be eligible for MRgFUS.
"We treated a smaller area using this device, yet still had very good results," study lead author Dr. Sangeet Ghai of the University Health Network Sinai Health and Women's College Hospital in Toronto said in the statement. "At the same time the patients preserved their erectile and urinary function."
Prostate cancer is traditionally treated with surgery and radiation therapy, which are effective but can leave patients with long-term complications such as incontinence and erectile dysfunction, the researchers wrote. Focused therapy can mitigate negative treatment effects, and in fact, this kind of technique using ultrasound has been used to treat other diseases.
But ultrasound doesn't image the prostate well enough to make a targeted approach viable, according to the authors. That's where MRgFUS could come in, although it is not yet approved for this application by the U.S. Food and Drug Administration (FDA), the team noted.
"By combining the high-intensity focused ultrasound device with MRI, we can target our treatment to the exact location, because we're able to pinpoint precisely where the tumor is," Ghai said.
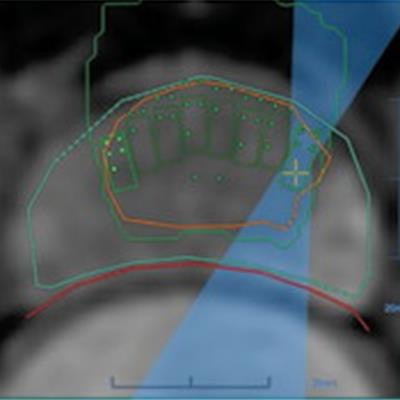
The investigators conducted a study that included 44 men with prostate cancer. All of the men underwent MRgFUS under general anesthesia, with the probe placed in the rectum to focus ultrasound waves at the cancer site. The team tracked patient outcomes after the procedure using MRI, biopsy, and erectile and urinary function surveys.
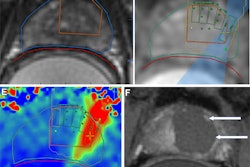
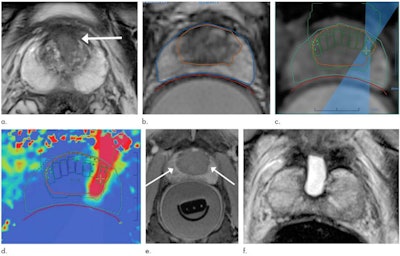 Images in a 69-year-old man with biopsy-confirmed prostate cancer classified as Gleason score 7 (3 + 4). (a) Pretreatment axial T2-weighted fast spin-echo MRI scan shows tumor in midline anterior transition zone (arrow). (b) Intraoperative MRI scan shows contoured rectal wall (red line), prostate margin (blue outline), and region of interest (orange outline). Because the urethra was included in the planned treatment volume, a suprapubic catheter was placed for continuous bladder drainage during treatment. (c) Intraoperative MRI scan shows focused ultrasound beam path (blue) overlaid on the treatment plan. Rectangles illustrate each sonication spot. (d) Thermal map image obtained during treatment with heat deposition color-coded in red overlaid on sonication spot. (e) Axial contrast-enhanced MRI scan obtained immediately after treatment shows devascularized ablated volume (arrows). (f) Corresponding T2-weighted fast spin-echo MRI scan at 5 months after ablation shows complete involution of the transition zone. All seven cores from treatment area margins were negative for cancer at biopsy. Images and caption courtesy of RSNA.
Images in a 69-year-old man with biopsy-confirmed prostate cancer classified as Gleason score 7 (3 + 4). (a) Pretreatment axial T2-weighted fast spin-echo MRI scan shows tumor in midline anterior transition zone (arrow). (b) Intraoperative MRI scan shows contoured rectal wall (red line), prostate margin (blue outline), and region of interest (orange outline). Because the urethra was included in the planned treatment volume, a suprapubic catheter was placed for continuous bladder drainage during treatment. (c) Intraoperative MRI scan shows focused ultrasound beam path (blue) overlaid on the treatment plan. Rectangles illustrate each sonication spot. (d) Thermal map image obtained during treatment with heat deposition color-coded in red overlaid on sonication spot. (e) Axial contrast-enhanced MRI scan obtained immediately after treatment shows devascularized ablated volume (arrows). (f) Corresponding T2-weighted fast spin-echo MRI scan at 5 months after ablation shows complete involution of the transition zone. All seven cores from treatment area margins were negative for cancer at biopsy. Images and caption courtesy of RSNA.Treatment was successful in all 44 men, and Ghai and colleagues found no procedure-related adverse events. Of the study cohort, 93% were disease-free at five-month follow-up. The men's scores for erectile function were similar at baseline and at five months postprocedure.
Although the study results are encouraging, using the technology regularly for prostate cancer treatment will require more research, according to an accompanying editorial written by Dr. Clare Tempany-Afdhal of Brigham and Women's Hospital in Boston.
"The next steps must be larger, multicenter trials ... [and] the MRI techniques must improve to allow for more accurate and greater tumor characterization," she wrote. "The road to full acceptance of this innovative approach remains challenging, with phase III trials and U.S. Food and Drug Administration, Centers for Medicare and Medicaid Services, and payor approvals all ahead ... [but] this study represents an important step forward in providing a noninvasive treatment for localized prostate cancer."



.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)



.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)











