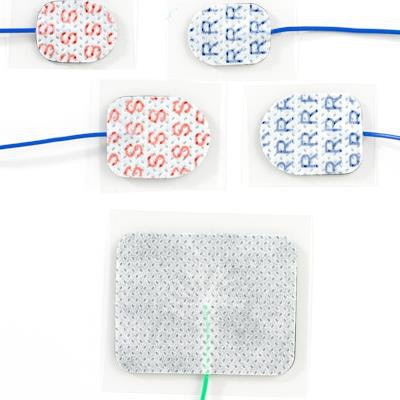
Medical device manufacturer Rhythmlink International said that its MR-conditional Sticky Pad surface electrodes have received U.S. Food and Drug Administration (FDA) clearance.
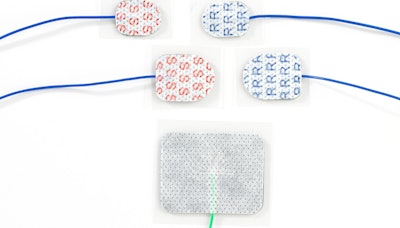 Rhythmlink's MR Conditional Sticky Pads are specially designed for stimulating, recording, or grounding. Image courtesy of Rhythmlink.
Rhythmlink's MR Conditional Sticky Pads are specially designed for stimulating, recording, or grounding. Image courtesy of Rhythmlink.As a result, the three varieties of the peel-and-stick electrodes can be utilized when patients need 1.5-tesla or 3-tesla MRI scans during intraoperative neurophysiological monitoring and electroencephalography surgeries, according to the vendor. The electrodes, which feature a self-adhesive hydrogel design, are each designed to provide either stimulating, grounding, or recording functionality, RhythmLink said.
All Sticky Pad electrodes can also be used during CT exams.


.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)




.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)











