MRI is often on the cutting edge of scientific innovation. But how are these innovations translated to the clinic? The process is complex, but the effort is worth it, according to a May 15 talk at the International Society for Magnetic Resonance in Medicine (ISMRM) 2021 and Society for MR Radiographers & Technologists (SMRT) virtual annual meeting.
"Translation of innovation to the clinic takes time -- it's not a five-minute job," said presenter Carolyn Mountford, PhD, of Griffith University in Queensland, Australia. Mountford is a researcher who specializes in MRI spectroscopic imaging and holds five patents in this area.
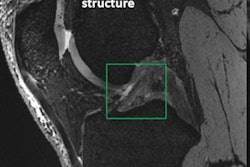
In her talk, Mountford outlined the steps for moving MRI research from the lab to the clinic using experience from her own projects as examples -- particularly work on identifying biomarkers for neurophysiological and mental health conditions (such as post-traumatic stress disorder or blast impacts to soldiers), developing an objective test for chronic pain, and evaluating cancer risk associated with breast density and methylmalonic acid (MMA) in healthy women.
At the start, researchers seeking to translate their discoveries into clinical use should consider the following questions:
- Is the innovation/clinical question relevant to patient management?
- Has the innovation been patented?
- Is the data sound?
- How will the technology be made available?
- How will clinical trial(s) be planned and undertaken?
- How will regulatory submissions to the U.S. Food and Drug Administration (FDA) be submitted and secured?
- Does the innovation/device meet FDA rules for breakthrough status?
- How will the new product be rolled out?
Patents are crucial, Mountford emphasized.
"You have to patent your innovation before you submit your abstract, before you even give a talk on it, and certainly before any paper goes to press," she said. "If one of these has occurred [before you have a patent] your innovation is in the public arena and you are not legally able to patent it."
A patent also protects against misuse of the technology, Mountford noted.
"If you have patented your innovation and someone chooses to use your technology and does it badly, you can stop them doing that," she said.
Mountford summarized four key tasks that must be completed before a new technology can be brought into clinical use, and stressed the importance of engaging expert assistance -- such as patent lawyers and clinical trial experts -- when needed to succeed.
- Patent registration: "Patent registration is a whole new language ... it's completely different from writing a manuscript," she noted.
- Clinical trials: "[We've seen] clinical trials experts save startup companies considerable amounts of money by pointing out they didn't have their trial planned appropriately," Mountford said.
- FDA submissions: "These require considerable experience knowing how to make the documentation, interface with FDA, so that the ... money spent on the clinical trial isn't wasted," she explained.
- Marketing
When it comes to funding for research innovation, there have been some positive changes over the past 20 years, according to Mountford. For example, in 2020, research translation and clinical trials were difficult to fund, but in 2021, there is more government and investor funding available.
In any case, investigators interested in bringing their science to the clinic need to be committed to the long haul.
"This is the process that one needs to go through," Mountford said. "It's difficult, but it's [important to] learn how to innovate, how to translate, and how to take an innovation all the way through to a product line."