French researchers have developed a new MR spectroscopy approach to study brain glucose metabolism, a process significantly involved in Alzheimer's disease. They presented their work in an animal study at the annual meeting of the International Society for Magnetic Resonance in Medicine.
In an MR research session on May 10 in London, Dr. Julien Flament of the Molecular Imaging Research Center in Fontenay-aux-Roses, discussed chemical exchange saturation transfer (CEST) findings in a brain study in rats using 11.7-tesla MR spectroscopy.
"This work illustrates the potential of gluco-CEST MRI as a tool to study brain energy metabolism and especially energy metabolism defects in neurodegenerative diseases," Flament said.
ISMRM 2022 was held in conjunction with the European Society for Magnetic Resonance in Medicine and Biology and the International Society for MR Radiographers and Technologists.
Impairing brain energy
Glucose hypometabolism is a decrease in glucose uptake in brain cells and is one of the main features of Alzheimer's disease, as it impairs brain energy in affected areas. Understanding how glucose is metabolized on a basic level could open new avenues to develop strategies to prevent neurodegeneration associated with the disease, Flament explained.
CEST is emerging as a new MRI contrast method for molecular imaging. The approach detects small amounts of contrast agent through saturation of fast-exchanging protons, and it benefits particularly from scanning at ultrahigh magnetic fields like 11.7 tesla. Gluco-CEST uses natural D-glucose as a contrast agent instead of conventional contrast agents, and thus provides data on glucose delivery, uptake, and metabolism in the brain.
The origin of the gluco-CEST-MRI signal remains an ongoing debate, Flament said. Some studies suggest an intracellular origin, while others conclude that the origin is vascular or extracellular, or both. In other words, quantifying cerebral glucose metabolism using the technique remains a hurdle.
"The origin of gluco-CEST signal remains unclear and must be elucidated," he said.
In this study, the researchers assessed a novel diffusion-weighted gluco-CEST MR spectroscopy approach and aimed for the first strong in vivo evidence that the origin of the glucose metabolism CEST signal is intracellular.
The group injected healthy rats with two glucose analogs: D-glucose, which is transported into intracellular spaces and quickly consumed to produce energy, and 2DG (2-deoxy-D-glucose), which is similarly transported, yet does not undergo glycolysis and instead accumulates intracellularly.
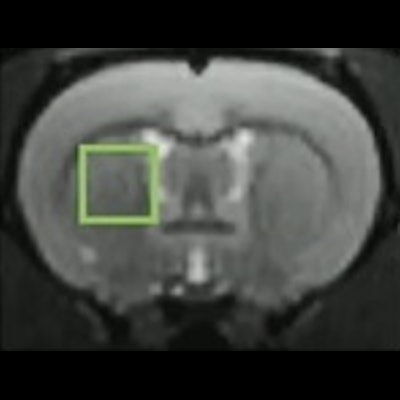
Imaging data were acquired using a horizontal 11.7-tesla MR spectroscopy Bruker magnet. Specifically, diffusion-weighted gluco-CEST data were acquired in a 2.5 x 2.5 x 2.5-mm3 voxel located in the left striatum of the rats over two hours before, during, and after injection.
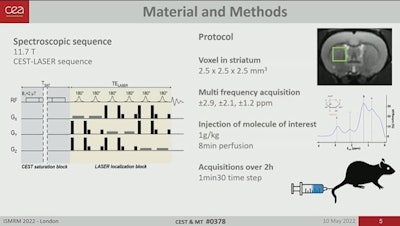 Image courtesy of Dr. Julien Flament.
Image courtesy of Dr. Julien Flament.The researchers observed that the gluco-CEST signal following D-glucose injection showed a small and continuous increase over time at each frequency of interest, while the gluco-CEST signal following 2DG injections showed a sharper increase followed by a plateau and a return to baseline.
Importantly, the researchers observed a marked difference between diffusion-weighted D-glucose and 2DG signals, Flament said.
Uptake of gluco-CEST signal after D-glucose injection was canceled with diffusion gradients, which suggests that the signal arises in these cases mostly from a "fast-moving space," namely the extravascular-extracellular space.
On the other hand, the gluco-CEST signal was preserved after 2DG injection: Kinetic features showed no difference with and without diffusion weighting, suggesting that molecules responsible for the 2DG gluco-CEST signal were mostly in "restricted space," or the intracellular space, Flament said.
"These observations argue in favor of an intracellular origin of CEST signal," he said.
Clinically, it may be some time before this technique can be compared to F-18 FDG PET, the gold standard in molecular imaging for assessing glucose hypometabolism in patients with Alzheimer's disease.
Yet the study could constitute a major step toward gluco-CEST quantification, and it could be beneficial for several applications, especially to study energy metabolism defects in neurodegenerative disorders, Flament concluded.