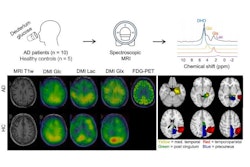
After decades of dormancy, deuterium is surging in research use as a contrast agent for MRI. Researchers discussed deuterium's potential in a talk at the International Society for Magnetic Resonance in Medicine (ISMRM) meeting.
In their presentations, Joseph Ackerman, PhD, from Washington University in St. Louis and Robin de Graaf, PhD, from Yale University talked about the history of deuterium and how it can be used as a safe, effective contrast agent in a method called deuterium metabolic imaging (DMI).
"It [DMI] provides unique imaging contrast that's not available with any other technique," de Graaf said. "It's easy to implement and really robust. I think it has a role in the clinic ... and the future looks bright."
The ISMRM held last week's meeting in conjunction with the European Society for Magnetic Resonance in Medicine and Biology and the International Society for MR Radiographers and Technologists.
'Heavy hydrogen'
Deuterium is a stable, nontoxic hydrogen isotope, sometimes called "heavy hydrogen." For radiology, deuterium metabolic imaging (DMI) can image active metabolism noninvasively for metabolic rate mapping or detecting unusual metabolism found in tumors or stroke cases.
Deuterium was first proposed for use as a contrast agent in 1982 and was used in vivo as a perfusion tracer for heavy water throughout the 1980s and 1990s. A 1987 research article also showed that deuterium resonances from metabolic products such as glucose and acetate can be observed in vivo. Previous research using animal models also showed that perfusion could be measured quantitatively and had high agreement with standard measures.
However, research on deuterium went mum as the world entered the 21st century. Ackermann said this was because of focus on proton MRI, which has high signal-to-noise resolution, speed, and multiple contrasts.
"Most MRI scanners were and still are only proton-enabled," he said.
Ackerman added that DMI could "greatly" benefit from ultrahigh-field scanners, which weren't available in the early days of deuterium's early research. However, these scanners are expensive and generally found at major MRI research centers.
De Graaf said DMI has strong potential to become a dominant MR research tool and imaging modality. He added that its advantages include high sensitivity, powerful acquisition methods, availability, and time efficiency.
He also echoed the sentiment of a 1992 quote by Dr. Robert London saying that the major advantage of using deuterium as an in vivo tracer is the "extreme technical ease" with which studies can be carried out.
"I think this is one of the reasons that deuterium metabolic imaging seems to be taking off. Almost any study will succeed," de Graaf said.
That takeoff, de Graaf said, was highlighted by studies in 2014 and 2017 showing DMI's high performance when used with high magnetic field scanners. Acquisition time for these studies took about one minute, but animal models were used.
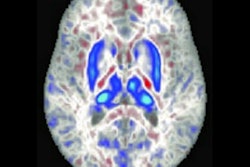
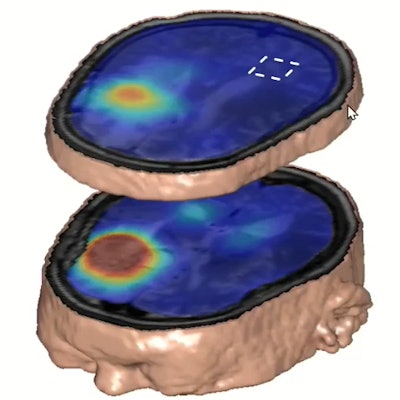
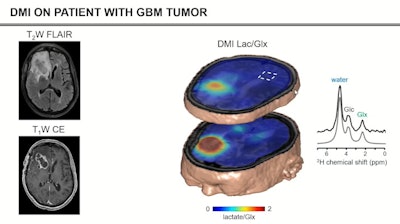 Deuterium metabolic imaging has made a reemergence in the research realm as an imaging modality that can be used with MRI. Researchers at the ISMRM meeting said it can be used as a safe, effective contrast agent to image parts of the body for tumor detection, such as the brain (pictured). Image courtesy of Robin de Graaf, PhD.
Deuterium metabolic imaging has made a reemergence in the research realm as an imaging modality that can be used with MRI. Researchers at the ISMRM meeting said it can be used as a safe, effective contrast agent to image parts of the body for tumor detection, such as the brain (pictured). Image courtesy of Robin de Graaf, PhD.However, in 2018, DMI's use on humans was demonstrated with a study of two patients showing imaging could be done in vivo. The study yielded 3D images of the human brain after patients consumed deuterated water containing glucose, glutamate, and lactate.
De Graaf also led studies showing DMI's performance across multiple magnetic fields and how they affect voxel size, a component of image quality. At a field strength of 4 tesla, DMI gives a voxel size of 8 mL, 3 ml at 7 tesla, and 2 ml at 9.4 tesla. With more conventional 3-tesla measurements, though, a voxel size of 14 ml is seen, though these results have been seen in healthy study cohorts.
"The volumes need to be slightly of course, but DMI has the potential, even at [3 tesla]," de Graaf said.
DMI can also be done in parallel with MRI, which could shorten image acquisition time from one hour, when the two are done back-to-back, to 30 minutes, he added.