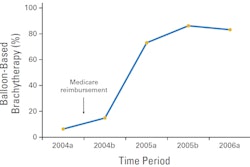
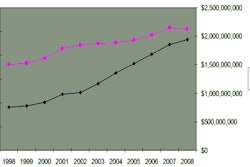
Image-guided therapy firm Brainlab has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its HybridArc radiosurgery planning software.
Designed to increase the efficiency of existing linear accelerator radiosurgery hardware, HybridArc can provide volumetric arc radiosurgery without the need for hardware upgrades, according to the vendor. The software employs an adaptive dose calculation matrix that considers the different parameters affecting dose distribution and calculates plans typically within a few minutes, Brainlab said.