Image guidance plays a vital role in modern radiotherapy, helping to reduce the impact of both interfraction and intrafraction motion. A session at the European Society for Therapeutic Radiology and Oncology (ESTRO) meeting, held last month in London, examined some recent developments in this field.
To account for interfractional tumor motion and deformation, imaging techniques such as conebeam CT (CBCT) are often employed to enhance the accuracy of patient alignment. However, CBCT also delivers radiation dose, both to the target itself and to nearby critical structures.
Jonathan Stenbeck, from South Carolina Oncology Associates, explained to ESTRO attendees how optimizing the CBCT within the treatment planning system can enable the imaging dose to be accounted for clinically. Stenbeck and colleagues at the University of South Carolina have demonstrated that it is possible to model the CBCT scan to estimate the dose that a patient will receive.
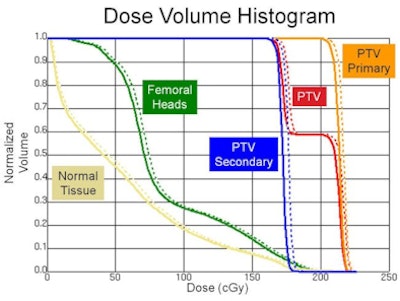
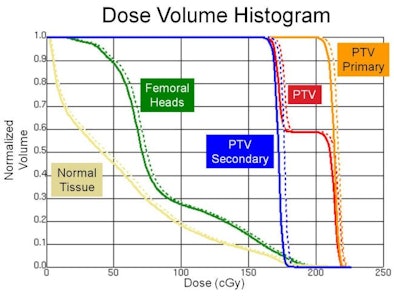 The above dose volume histogram shows the dose to regions of interest from an intensity-modulated radiation therapy (IMRT) plan (solid line) and the IMRT plan with CBCT included (dashed line).
The above dose volume histogram shows the dose to regions of interest from an intensity-modulated radiation therapy (IMRT) plan (solid line) and the IMRT plan with CBCT included (dashed line).Using a Syngery linac (Elekta) equipped with x-ray volume imaging (CBCT), the researchers measured the dose from a 120-kVp beam at various depths in a water tank. Ion chamber measurements were used to obtain depth dose curves and beam profiles (averaged over five scans), and the resulting data were used as the input into a Pinnacle Beta v9.1 treatment planning system (Philips Healthcare).
Although treatment planning systems typically cannot model photon beams at the low energies used by CBCT (less than 150 kVp), by introducing monoenergetic energy deposition kernels from 20 to 110 keV into the planning system, Stenbeck and colleagues were able to model the diagnostic energy beam.
The researchers then created a Pinnacle CBCT plan for a pelvic phantom loaded with thermoluminescent dosimeters (TLDs). The TLD-measured dose agreed well with the dose predicted using the model. "Now that we trust our model, we can use it in the treatment planning system to look at the dose-volume histograms," said Stenbeck. He noted that in this phantom, dose to the planning target volume was seen to increase by 1.9% due to the CBCT scans, while dose to the femoral head and to normal tissue increased by 3.8% and 5.7%, respectively.
Stenbeck concluded that it is possible to model the dose received from CBCT in a treatment planning system. Future work will include testing the use of shielding and reduced scanning angles for dose reduction, as well as expanding the study to the Oncentra (Nucletron) treatment planning system.
Intrafraction imaging
Martin Fast, from the German Cancer Research Center (DKFZ), addressed the issue of intrafractional motion. He described an x-ray imaging framework developed at DKFZ that monitors target location throughout a treatment fraction, either continuously or on demand.
 The experimental setup showing the in-line geometry, with vertical alignment of the kV and MV beam axes.
The experimental setup showing the in-line geometry, with vertical alignment of the kV and MV beam axes.
The imaging equipment employs an in-line geometry, in which the kilovoltage (kV) imaging and megavoltage (MV) treatment beam axes are vertically aligned, with a silicon flat-panel detector mounted underneath the treatment head. This unique geometry enables monitoring of motion perpendicular to the treatment beam, the direction that usually features steep dose gradients.
However, the fact that the MV and kV signals exist in the flat-panel detector at the same time does throw up some challenges. "The primary radiation from the MV source and from scatter is much higher than the kV signal," said Fast. He noted that, as well as causing a central blind spot, MV ghosting and stripe artifacts can also occur.
To address these issues, the researchers implemented three design features. First, they retracted the kV source toward the gantry by 13 cm. This intentional misalignment geometrically separates the MV treatment field and kV image on the detector and maximizes the quality of the kV image.
Second, the researchers employed MV signal subtraction. This involves acquiring an MV image, immediately followed by the MV plus kV image, and then performing a subtraction to generate a kV-only image. Fast noted that this process enabled visualization of markers that couldn't be seen in the combined image.
Finally, to maximize the kV-to-MV signal ratio, the kV beam is only triggered when needed (in contrast to the MV source, which is always on during treatment), and the flat-panel detector read-out triggered simultaneously with the kV shot. The final kV images are analyzed with an automated threshold-based marker detection algorithm and the positional information sent to a tracking tool to enable any required adjustments.
The DKFZ researchers evaluated the imaging system using a moving phantom with metallic markers. With the above adaptations, the system delivered a latency of 89.23 msec (compared to 86.7 msec with the MV beam switched off) and an average position accuracy of 0.23 mm (0.57 mm maximum).
"We have an in-line imaging system that can talk to the DKFZ tracking tool," said Fast. "It can get marker position every 140 msec with an accuracy of better than 0.5 mm." He noted that the overall imaging system latency was 160 msec or less. Image acquisition and postprocessing account for about 90 msec of this, implying that the mean age of the reported position is 70 msec.
© IOP Publishing Limited. Republished with permission from medicalphysicsweb, a community Web site covering fundamental research and emerging technologies in medical imaging and radiation therapy.



















