Radiation therapy firm Varian Medical Systems has received clearance from the U.S. Food and Drug Administration (FDA) for its Pivotal Care Solution for prone breast treatment, which is designed to treat patients lying on their stomachs rather than their backs.
For many women without axillary lymph node disease, treatment in the prone position can significantly reduce the volume of lung and heart tissue exposed to the treatment beam, Varian cited from recent studies.
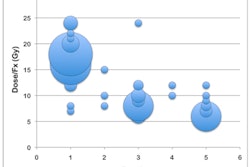
In other Varian news, the company has added updated control software to its Clinac and Trilogy linear accelerators. The software is designed to enable delivery rates of up to 2,400 monitor units per minute -- double the previous highest output -- to provide higher doses in just one or a few treatment sessions for tumor ablation.