The Radiation Therapy Oncology Group (RTOG) has begun a phase I trial to test the monoclonal antibody ganitumab for locally advanced pancreatic cancer.
Ganitumab is a fully-human monoclonal antibody antagonist of the insulinlike growth factor-1 receptor, the group said. The trial, RTOG 1102, is expected to provide dosing and safety information needed before advancing ganitumab to a phase II randomized trial.
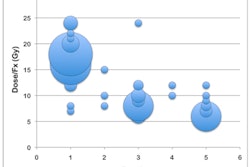
Up to 42 study participants at institutions across the U.S. will be enrolled in the trial and will receive two months of induction chemotherapy with gemcitabine and ganitumab to provide early systemic treatment, as well as select the study participants most likely to benefit from chemoradiation, RTOG said.
Dr. Christopher Crane of MD Anderson Cancer Center at the University of Texas will serve as principal investigator for the trial.