Oncology firm Elekta has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for an autoscan option that allows remote, automated ultrasound scanning with Elekta's Clarity soft-tissue visualization system.
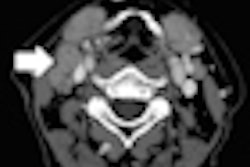
The option enables ultrasound scanning of the prostate and surrounding anatomy to be performed outside a treatment room using a motorized probe positioned at a patient's perineum.
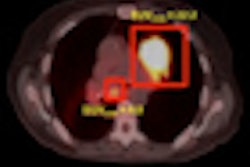
With a transperineal approach, the Clarity system provides a clear view of the prostate and penile bulb, which facilitates treatment planning, according to the company. The functionality could help physicians create treatment plans with tighter margins around intended targets, preventing exposure to uninvolved tissues.
Prior to receiving FDA clearance, the product was evaluated as a radiotherapy imaging tool in clinical trials at Fletcher Allen in Vermont. Radiation oncologists there will use Clarity with autoscan to track prostate motion in real-time during treatment, facilitating on-the-fly adjustments to a patient's position, the company said.