An ultrashort course of accelerated partial-breast irradiation (APBI) using a balloon device is achievable and well-tolerated by patients, according to results from a prospective, phase II clinical trial presented at the San Antonio Breast Cancer Symposium held last week.
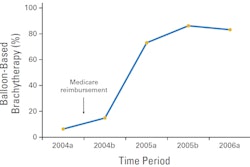
Ultrashort APBI falls between single-dose intraoperative irradiation of the breast cavity immediately following a lumpectomy, and conventional APBI consisting of 10 doses delivered in five days. This poster presentation described six-month outcomes of patients who received four radiation doses of 7 Gy.
The single-arm, multi-institutional phase II trial is designed to sequentially treat three cohorts of women with three progressively hypofractionated schedules. The women are 50 years of age or older with unifocal invasive or in situ tumors 3 cm or smaller, excised with negative margins. The women also have negative lymph node involvement and are hormone-receptor positive.
In this cohort, 30 patients received treatment twice daily using a multilumen breast brachytherapy balloon (Contura, SenoRx). The median skin dose as a percent of prescription dose was 84% and the median rib dose was 71%.
Toxicities were minor and resolved with follow up or minimal intervention, lead researcher Dr. Atif Khan, a radiation oncologist at the Cancer Institute of New Jersey, and colleagues found. One breast infection, two cases of symptomatic fat necrosis, and two cases of symptomatic seromas occurred. No acute toxicities were greater than grade 2.
Researchers at the Cancer Institute of New Jersey have opened enrollment for the next part of the study, which will evaluate a three-dose schedule of 8.25 Gy delivered in two days. The goal is to reach a 10.25-Gy radiation dose delivered over a two-day period.