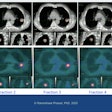
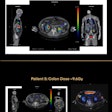
Radiation therapy firm Varian Medical Systems has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its updated ProBeam proton therapy system.
ProBeam gives clinicians options for minimizing dose to healthy tissue in the course of delivering proton therapy treatments for cancer. Its scanning beam technology enables intensity-modulated proton therapy (IMPT) by modulating dose levels on a spot-by-spot basis throughout the treatment area, according to the firm.
Varian's scanning beam IMPT technology is already being used in the Rinecker Proton Therapy Center in Germany, which has now treated more than 1,500 patients. Varian also has contracts to install ProBeam systems at six sites: four in the U.S., one in Saudi Arabia, and one in Russia, the company said.