Prostate radiation therapy device developer Augmenix is highlighting the results of a study of its Spacing Organs at Risk (SpaceOAR) product in improving the quality of life for patients undergoing prostate radiation therapy.
SpaceOAR is injected as a liquid that then solidifies into a soft hydrogel that pushes the rectum out of the high-dose prostate radiation field for three months during radiation therapy. It is designed to temporarily position the anterior rectal wall away from the prostate during prostate cancer radiation therapy; by creating this space, SpaceOAR reduces radiation dose delivered to the anterior rectum, according to the company.
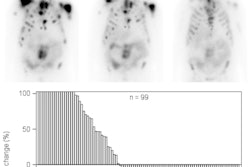
In the randomized phase III trial, 149 patients received SpaceOAR and 73 were control patients. At the three-year time point, grade 1+ rectal toxicity was decreased by 75% in the SpaceOAR arm, and no grade 2+ rectal toxicity was seen with the spacer. In addition, those who received SpaceOAR had less of a decrease in urinary and bowel quality of life, compared with the control group.
The long-term outcomes data were published December 22 in the International Journal of Radiation Oncology, Biology, Physics. Previously, only the use of 3D-conformal therapy had been tested in such a phase III trial, but both image-guided radiation therapy (IGRT) and intensity-modulated radiation therapy (IMRT) were routinely adopted anyway on the basis of less evidence, wrote lead author Dr. Daniel Hamstra, PhD, and colleagues.