German researchers are highlighting the potential of a new therapeutic agent for radioligand therapy of patients with metastatic castration-resistant prostate cancer in a study published in the January issue of the Journal of Nuclear Medicine.
The clinical trial so far has used lutetium-177 (Lu-177) prostate-specific membrane antigen 617 (PSMA-617) on 145 patients at 12 therapy centers across Germany. Some side effects have been reported, but the researchers have deemed them manageable (JNM, Vol. 58:1, pp. 85-90).
PSMA is abundant in prostate cancer and even more prevalent in the castration-resistant form of the disease, which has a median survival rate of less than two years.
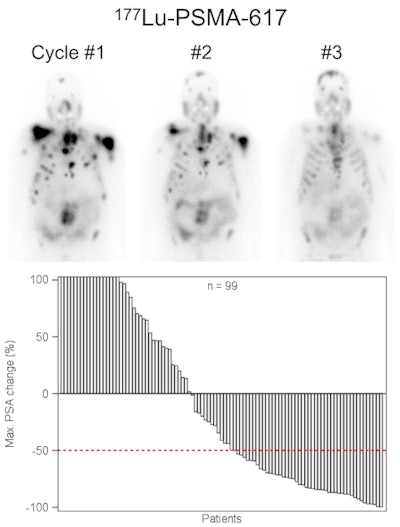 Lutetium-177 PSMA-617 radionuclide therapy has been shown to reduce tumor burden and induce prostate-specific antigen remission in patients with metastatic advanced prostate cancer. Image courtesy of JNM.
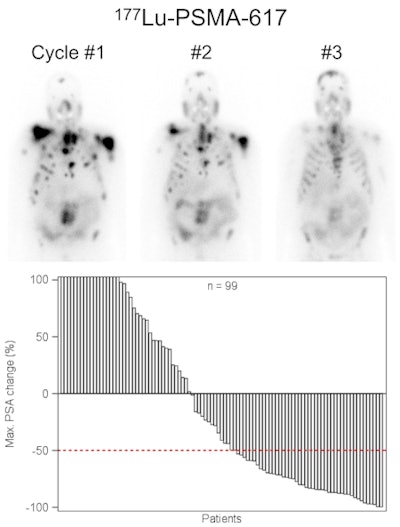
Lutetium-177 PSMA-617 radionuclide therapy has been shown to reduce tumor burden and induce prostate-specific antigen remission in patients with metastatic advanced prostate cancer. Image courtesy of JNM.The 145 subjects (median age, 73 years; range, 43-88 years) were treated with Lu-177 PSMA-617 between February 2014 and July 2015 with one to four therapy cycles. A total of 248 therapy cycles were performed on the patients.
Overall, 45% of the patients had a positive response after all therapy cycles, while 40% responded positively after a single cycle. In terms of side effects, 18 patients had hematotoxicity; 10% had anemia, 4% experienced thrombocytopenia, and 3% had leukopenia. In addition 8% of patients experienced dry mouth.
Future phase II/III studies are needed to clarify the survival benefit of the new therapy for this type of patient, concluded lead author Dr. Kambiz Rahbar and colleagues.