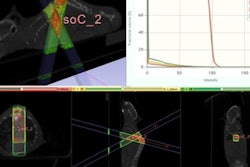
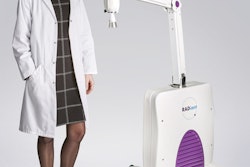
X-ray and radiation therapy technology developer Xstrahl has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its Photoelectric Therapy System, an x-ray therapy system designed to treat nonmelanoma skin cancer and superficial lesions.
The Photoelectric Therapy System operates in the 10-kV to 80-kV range and is intended for superficial radiotherapy and surface electronic brachytherapy treatment of primary malignant epithelial neoplasms of the skin and keloids, Xstrahl said.