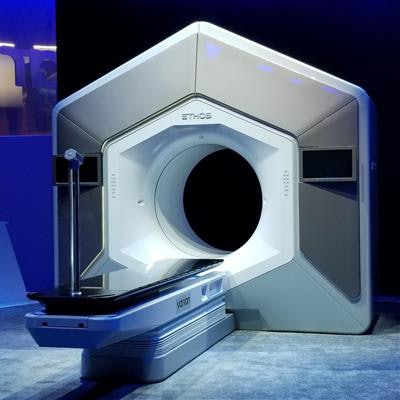
Radiation therapy firm Varian Medical Systems has garnered U.S. Food and Drug Administration (FDA) clearance for its Ethos system, a radiation therapy platform that utilizes artificial intelligence (AI) to adapt treatments in real-time to changes in tumors and patient anatomy.
Ethos was first introduced at the 2019 American Society for Radiation Oncology meeting in September and is the first Varian system to rely on AI to guide treatment. It can provide an entire adaptive treatment in a typical 15-minute time slot, including patient setup through treatment delivery, according to the vendor.
With Ethos, physicians first define their clinical intent using predefined templates, which are used to generate initial treatment plans. An onboard conebeam CT (CBCT) scanner then acquires high-resolution images in about 17 seconds before treatment begins. At that point, the system's AI algorithm deploys a deformable image registration technique to compare the CBCT scans with reference images from other modalities that were acquired during treatment planning.
If the target tumor has shifted or if there are changes in patient anatomy between when the onboard scans and the planning images were acquired, Ethos' AI algorithm is able to make modifications to the treatment plan while the patient is still on the couch, according to the company. After a quick quality assurance check, the modified treatment is delivered -- without delaying the treatment session. If nothing changes, the session continues as planned.


















