The U.S. Food and Drug Administration (FDA) has cleared artificial intelligence (AI) firm Vysioneer's brain tumor autocontouring software, called VBrain.
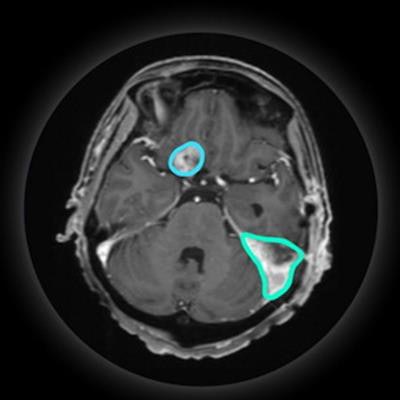
VBrain maps three types of brain tumors -- metastasis, meningioma, and acoustic neuroma -- allowing patients to begin radiation therapy more quickly than when contouring must be performed manually, the company said.
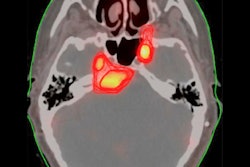
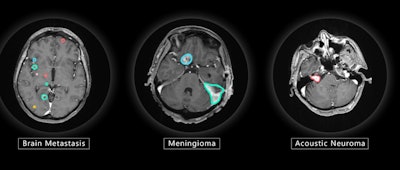 VBrain applies autocontouring to the three most common types of brain tumors: brain metastasis, meningioma, and acoustic neuroma. Image courtesy of Vysioneer.
VBrain applies autocontouring to the three most common types of brain tumors: brain metastasis, meningioma, and acoustic neuroma. Image courtesy of Vysioneer.In an 18-month clinical trial conducted at the National Taiwan University Hospital in Taipei, researchers found that VBrain had a 12.2% higher sensitivity for detecting lesions compared with manual contouring, and it decreased treatment planning time by 30.8%, Vysioneer said.