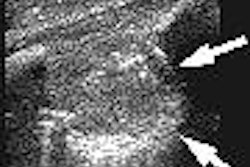
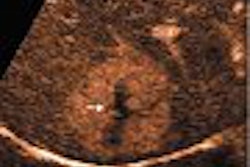
Imaging software developer Cedara Software, a division of Merge Technologies of Milwaukee, announced that the breast computer-aided detection (CAD) system, Cedara B-CAD, has received Food and Drug Administration 510(k) clearance. The system is designed to help radiologists analyze breast ultrasound images through automated segmentation, characterization, classification, annotation, and report generation.
Cedara B-CAD was developed by Toronto-based Medipattern, a company specializing in medical pattern recognition, and is being marketed by Cedara through its technology partnership program. Breast ultrasound is particularly useful for evaluating palpable masses in young (under 30), pregnant, or lactating women, among others, according to the company.
Cedara B-CAD will soon be commercially available in the U.S., the company said.
By AuntMinnie.com staff writers
June 2, 2005
Related Reading
Merge eFilm, Cedara close deal, June 1, 2005
Court approves Cedara merger with Merge, May 27, 2005
Merge shareholders approve Cedara merger, May 25, 2005
Cedara posts record Q3, April 28, 2005
Analogic divests Cedara stake, February 18, 2005
Copyright © 2005 AuntMinnie.com