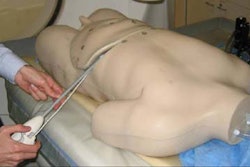
Ultrasound developer Misonix has received a Canadian Device License for its ultrasonic wound debridement system.
The regulatory approval in Canada follows the announcement earlier this month that Misonix had received clearance on its 510(k) application with the Food and Drug Administration to market the product for wound debridement, according to the Farmingdale, NY vendor.
By AuntMinnie.com staff writers
June 28, 2005
Related Reading
Misonix, University of Pittsburgh to test 'bone cutter', June 3, 2005
Misonix files 510(k) for ultrasonic wound debrider, April 21, 2005
Misonix adds to executive ranks, September 28, 2004
Misonix expands into Latin America, April 13, 2004
Misonix brings Sonablate to Europe, March 31, 2004
Copyright © 2005 AuntMinnie.com