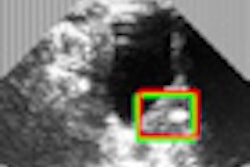
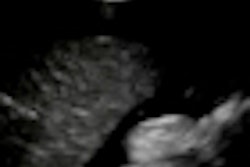
The International Contrast Ultrasound Society (ICUS) has submitted a citizen petition with the U.S. Food and Drug Administration (FDA), calling for removal of black box warnings on ultrasound contrast agents.
In making its case, ICUS said that a significant body of new research shows a favorable cost-benefit ratio for contrast-enhanced ultrasound; this research was not available when the boxed warnings were mandated by the FDA, according to the ICUS petition. The warnings deter use of a safe, inexpensive, and radiation-free diagnostic imaging tool with potential lifesaving benefits for patients, ICUS said.
Recent studies now show no increased safety concerns for ultrasound contrast, even among the sickest patients, according to the ICUS petition. It cited six new safety studies undertaken by product sponsors at the request of the FDA, as well as numerous other independent studies recently published in peer-reviewed journals.
The society voted at its annual conference and board meeting in September to formally request FDA action on the black box warning.