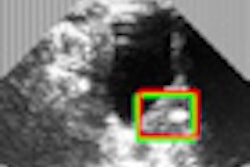
Ultrasound accessories firm CS Medical has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its TD-100 automated transesophageal ultrasound probe disinfector.
Utilizing a microprocessor controller, TD-100 provides a user interface that guides the technician through the few steps of the disinfection that require user input, according to the firm. Once initiated, the disinfection process continues automatically and alerts the technician at the end of the 17-minute cycle, CS Medical said.