The U.S. Food and Drug Administration (FDA) has cleared GE Healthcare's latest version of its Vivid E9 cardiovascular ultrasound system.
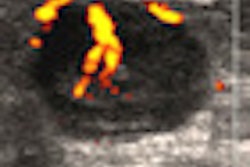
Vivid E9 Breakthrough 2012 (BT12) includes a 4D transducer for transesophageal echocardiography (TEE). This transducer allows clinicians to view images of the heart during assessment and diagnosis performed in the echo lab, and it supports invasive surgical procedures in the operating room, according to GE.
With the TEE transducer, Vivid E9 can also be used for procedures such as mitral valve repair, transcatheter aortic valve repair, atrial septal defect closures, and patent foramen ovale closures, the company said.
Vivid E9 BT12 has the CE Mark and has been available for sale in Europe and other countries around the world.