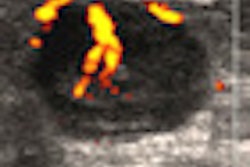
The U.S. Food and Drug Administration (FDA) has released an update on the risk of bacterial infection from Other-Sonic generic ultrasound transmission gel manufactured by Pharmaceutical Innovations.
In April, the FDA issued a safety communication to alert healthcare professionals who perform ultrasound procedures to stop using Other-Sonic gel manufactured from June to December 2011 due to the risk of infection from two strains of bacteria, Pseudomonas aeruginosa and Klebsiella oxytoca. U.S. marshals acting at the request of the FDA also seized all lots of the company's gel that was manufactured during the period.
In an updated safety communication released on Friday, the FDA said that Pharmaceutical Innovations has now included lot numbers 060111, 090111, 080111, and 1009111 in its recall. While the agency said it continues to also advise healthcare providers to stop using products from lot 120111, it said that Pharmaceutical Innovations told the FDA that lot 120111 was never distributed to U.S. customers and was therefore not part of its voluntary recall.