Ultrasound technology start-up Echometrix has received U.S. Food and Drug Administration (FDA) clearance for its EchoSoft musculoskeletal ultrasound software package.
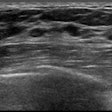
Designed to be used with diagnostic ultrasound to help diagnose and monitor musculoskeletal conditions, EchoSoft generates color maps to help clinicians visualize structural characteristics that are unique to tendons and ligaments, according to the firm.
EchoSoft can be used with ultrasound equipment produced by all major manufacturers, Echometrix said. The company continues to participate in longitudinal clinical studies with partners in the U.S., Canada, and Australia.