AuntMinnie.com presents the 25th in a series of columns on the practice of ultrasound from Dr. Jason Birnholz, one of the pioneers of the modality.
This is my 25th entry in this sporadic series of columns that intend to be helpful, to explore issues that are outside the usual realm of ultrasound publications or presentations, and to prod the boundaries of routine clinical practice. I am very thankful and appreciative of the editors of AuntMinnie.com, who have always been completely supportive.
 Dr. Jason Birnholz.
Dr. Jason Birnholz.My articles can often be like crawling through Swiss cheese. There is an entry point, lots of side channels, occasions for appreciating the scenery -- repetitive though it might be -- and, sometimes, a discrete exit. That's the way that diagnosis works in imaging. The starting point can be just a thread, an observation, a wisp of suspicion that we unravel, trying to find a lurking form of pathology or pathophysiology that leads to an informational exit. It's a probabilistic realm of shifting sands, where there are few handholds or places to step safely.
I often add something about myself or my experiences in these columns, obviously intentionally. When we deal directly with patients (which ought to be everyone who does diagnostic ultrasound), our personas come into play. That would be the total of our outlooks, experiences, education, personality, intelligence, and emotions, plus everything else.
The mix always includes covert biases. What physicians all learn is to recognize and analyze our instant impressions on meeting a new patient and counteract anything that will dampen our respect for that individual or impede providing our best care for him or her. If this is impossible, our duty is to find someone else to step in.
We work with our emotions, yet we also evaluate data with an equally absolute dispassion. The self should never come into our acquisition procedures and analysis of data, or appear in a scientific publication. It's foolish to ask if medicine is an art or science. It's both, all the time, just like the quantum duality of photons as waves and particles; it is what it has to be in each epoch of individual medical care.
Visiting a podiatrist
The starting point for this meander was a hospital social event. I was queued up, plate in hand, ogling a bowl of shrimp, when I was joined by a podiatrist I had not seen in many years. We had lived in the same school district once. We chatted about our children, or at least as much as we could in the din. Like any proud parent, we were simultaneously in the present and back in past years when they were in grade school.
He asked me if I knew anything about ultrasound. I said, "a bit," and he mentioned that he had been doing ultrasound in his office for several years and found it invaluable. He added, "It's all about evidence-based medicine."
I thought that was an incredible observation, and I arranged to visit his office to learn more. I thought this was a good opportunity to study two excellent, back-to-back papers published earlier this year in RadioGraphics that I had previously neglected, as musculoskeletal applications had never been my primary interest. An Italian research team led by Dr. Luca Maria Sconfienza detailed dynamic high-resolution ultrasound of ankle and midfoot ligaments, while another group led by Dr. Mihra Taljanovic, PhD, of the University of Arizona Health Network described high-resolution ultrasound and MRI of peroneal tendon injuries.
I expected to learn a lot from my visit, and, indeed, the experience turned out to be eye-opening. My host was gracious and had me watch a few exams. I saw no ultrasound-related materials of any kind in his office. He said he had learned all about ultrasound on his own, plus a lot from the salesperson who had sold him the equipment and later installed it. Ultrasound was not part of the curriculum in his day, and there also wasn't any requirement for residency training before licensure and going into practice.
Well, I mostly taught myself when I had my first B-scan unit loaned to me at the U.S. National Institutes of Health (NIH) in the 1960s, so I could relate. He said his equipment was old but completely optimized for podiatry. That seemed to mean foot-related annotations. There was a printer, but apparently no option for saving the image file. There was no use or knowledge of Doppler, and none of the controls had apparently ever been altered since installation.
Patients were prone with their legs over the edge of the exam table and the operator was seated in front of their feet. A long-axis image of the plantar fascia was frozen, annotated, and its maximal thickness determined by a cursor on the display screen. He also aligned the transducer along the big toe and gently distracted it while watching the joint space. There was probably more, but that's all I recall. I asked about guidance for injection in treating plantar fasciitis. He held the transducer along the sole of the foot and described the needle path from the side of the heel, with the needle appearing as a central dot as it crossed the scan plane.
I assume that the podiatrist thought that ultrasound was child's play. He did not know or care about any other ultrasound application or the technical aspects of ultrasound imaging. I'm pretty sure he assumed that I could not know anything about feet. He didn't ask me for any advice about operating the equipment, and I didn't volunteer anything, although I couldn't resist adjusting the analog video display's brightness and contrast, which were really far off of optimal. I took this out of an ultrasound equipment context by saying that this was something I always had to do with an old TV at home. My friend was proud of his skill, and it was plain that he cared for his patients.
Plantar fasciitis screening
After I asked him about "evidence-based medicine," he gave me a disapproving and disappointed look and said that ultrasound gave him a way to provide evidence to insurance carriers of having done an exam or procedure and for substantiating a diagnosis, so that he would be reimbursed. He added that he had started screening for plantar fasciitis in his patients without heel pain via fascia thickness and that he had been able to uncover many undiagnosed cases.
I was astonished by this interpretation of "evidence-based medicine," but I could understand this usage in a context of reimbursement. I did some Web searching under "podiatric ultrasound," and one of the top links was to an ultrasound equipment distributor with entries such as "no training necessary" and "be up and running instantly." There were specific International Classification of Diseases (ICD) coding instructions, recommended exam pricing, and estimates of income increases from adding ultrasound to a podiatry office practice. I guess they had the same understanding of evidence-based medicine.
There is something admirable, though, in my friend's use of an available tool to find a subacute form of a disease. Plantar fasciitis is highly prevalent, but it is usually self-limited. It is the most common cause of heel pain, but not the only cause by a long shot. I found one meta-analysis that indicated a thickness measurement greater than 5 mm could be taken as evidence of abnormality.
Plantar fascia thickness increases with weight and with age, as well as with the daily amount of walking and running. When there is plantar fasciitis, as diagnosed by heel pain on walking and with dorsiflexion of the toes, the first thing to do is switch whatever footwear is used to something that cushions and absorbs shocks. It is reasonable to presume that repeated trauma is involved and that plantar fascia thickens as a compensatory mechanism, which would make this a secondary finding, even though it is treated as the problem itself by my friend and in the podiatry literature.
Two odd and very old factoids popped up in my memory when I started thinking about feet for this article. I tracked down a reference for one out of curiosity. In walking, the arch is raised by tightening of the plantar aponeurosis, apparently not by muscle activity, according to this 1954 article in the Journal of Anatomy. The other was that fat pads deep to the fascia have an unusual lipid composition that may promote shock absorption.
Here's a hypothesis: Repeated stress from pounding on the fascia followed by its stretching promotes fibrosis thickening, while injury to the underlying fat pads releases proinflammatory cytokines, initiating inflammation. I don't know if this is true, and I don't expect to revisit this topic. The concept is that there are two ways to go about ultrasound: One is very limited and seeks signs, while the other bases what we do on a model of what we want to identify. I will come back to this in a bit; it is very important.
Basic equipment adjustments
My podiatrist friend was mainly using ultrasound as a lab test with a numerical result, not particularly different from the earliest A-mode determinations of fetal biparietal diameter (BPD) at term. The imaging part of the exam was trivial; most of the available information was ignored or unavailable, as visualization was limited to maximally "contrasty" objects. I suspect that this may be common in many of the practice areas that utilize ultrasound clinically without fully dissecting and optimizing the entire image chain.
The eye and the brain are part of any imaging chain. We look at images on a screen or on some display medium. The primacy of visual perception in the interpretive process is so fundamental that it's easy to overlook.
I always used to teach residents and fellows that they needed to adjust the equipment before they tried to look at any details of the image. I set them up with the phantom de jour and had them turn every knob, slide every slider, and flick every switch to see what it does to the image. Individual changes are pretty easy to assess. They had to look at combinations of simultaneous settings, which is a lot harder.
Ultimately, I had them set up equipment for scanning people; the only criterion was that the settings were optimal for diagnosing (or excluding) the particular pathology that was being sought. Mostly, this meant decreasing random noise and maximizing contrast for the lesion or target tissue component versus the background. There was no discrete "right" setting. Adjustments were tweaked or varied continuously throughout the exam and transducers were changed as needed. This approach is generic in that it applies to whatever equipment is used and whatever the clinical task.
Beginners focus on spatial resolution; however, our ability to visualize pathology in most applications depends on contrast resolution. Image persistence, dynamic range, grayscale display maps, and transducer choice are contrast adjustments available to the operator. The issue of contrast resolution is complicated, as it involves all of the factors governing the transmission and reception of tailored acoustic pulses, which are equipment-specific and typically inaccessible.
Some of you might say something like, "Why, with modern equipment, I never have to adjust anything. There are presets for every organ and every application." The problem with that approach, though, is that you are relegating your ability to optimize the exam for your patient and his or her clinical problem to an unknown person's choice of what they think is best for some ideal -- and often normal -- test target.
A preset or a no-set might be applicable for a very simple and limited imaging task, like the podiatry example, but it is restrictive and limiting for the more general task of searching for an unknown in a sea of multiple possibilities. There are automated one-click optimization schemes, but these tend to lack the flexibility required for dealing with diverse clinical scenarios. This is a facet of scanning that I will be investigating at the RSNA meeting next week.
Old controversies
At the start of grayscale imaging, some of the early adopters displayed images as black on white. TV display devices usually had an option for display phase. There wasn't much grayscale in the images. Even so, you could not make a worse choice perceptually. This has been standard practice for decades, and most units lacked this adjustment altogether.
However, a few early and influential people who had initially championed white on black opted for images of parenchyma that were smooth and continuous. Their rationale was that a solid organ should be filled with echoes, so they filled in parenchymal fields by pumping up signal compression to 70 dB or 80 dB. It may make liver images pretty, but the downside is that noise is high while contrast is low.
Consequently, it's really hard to identify focal lesions such as metastases and hepatomas or even to see intrahepatic branch vessels. Their comment was usually something like, "We have CT (and later, MRI) for those tasks, so it doesn't matter."
Ultrasound had a major advance, though, when my once-upon-a-time fellow and exemplary colleague Dr. Beryl Benacerraf published in 1985 the first series linking nuchal skin elevation to aneuploidy, specifically Down syndrome.
There is an interesting bit of history with that research. Those same pundits that were proud of their smooth parenchymal fields made some vicious, public denunciations of the sign because they could not replicate it themselves. Beryl's first series was based on almost 1,000 consecutive amniocenteses and ultrasound exams, which should have made it incontrovertible. The only explanation I could come up with was that perhaps there was some difference in the phenotypic expression of Down syndrome on the two coasts of the U.S., which were the main venues of the controversy. I chose to respect their technical expertise for ultrasound.
Later, I came to realize that the West Coasters were adjusting their equipment on aesthetic grounds, not in terms of pathology recognition. Dynamic range settings were high (not the 45 dB to 50 dB ones best for visual contrast perception), and they tended to use sigmoidal postscan grayscale allocations, further limiting contrast. There was so much noise that the sign was obscured. Endovaginal scanning is an inherently lower-noise portal than a transabdominal one, so even with bad instrument settings, the sign was at least perceptible most of the time. This, possibly more than anything else, prompted appreciation of Beryl's work.
There have been programs that "certify" proficiency in nuchal translucency (NT) for several years. If you know how to scan fetuses, then a certification for a sign is absurd. If you don't, however, and you want to use ultrasound as a tool for that sign, then certification is fine, but you probably shouldn't be scanning anything else. I wonder if the courses on NT ever address the issue of adjusting equipment, including transducer selection, for this task.
There are still some facilities that offer NT testing to screen for risk of aneuploidy. I also wonder if those places are using the podiatrist's meaning of "evidence-based" without paying attention to the overwhelming peer-reviewed literature concerning cell-free DNA. A bit of old-time radiology wisdom from the plain-film era goes: "A sign is not the disease, the absence of a sign is not a sign." A modern codicil is that imaging signs from naked eye-level observations will have a limited lifespan as molecular foundations for diseases are understood and more-refined tests are developed.
3D faces
I started doing surface-rendered 3D images with software provided by TomTec Imaging Systems more than 10 years ago. This utilized manual sliding or rocking the transducer, so that I could use any transducer and could scan slowly for best sampling of the target volume. Right at the start, it was obvious that our usual fetal settings were fine for problems such as facial clefts, but, being low in noise, they were unsuitable as presents for prospective parents, who have every right to expect a baby's skin to appear smooth, continuous, and perfect.
The solution was to adjust the equipment for a visually aesthetic result for gift images. The example below is a 14-week fetus scanned transabdominally with a 15-MHz probe. Incidentally, there are essentially no facial expressions at this stage. The cheeks are one of the earliest places where white fat is deposited.
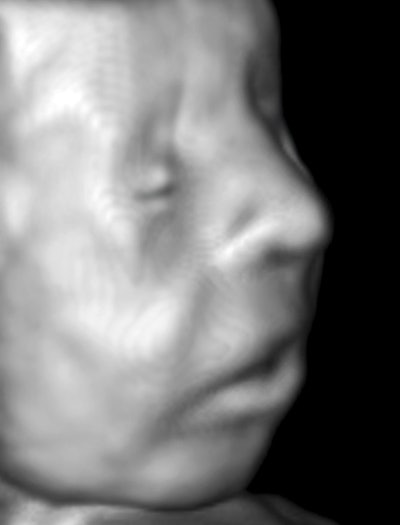 Image courtesy of Dr. Jason Birnholz.
Image courtesy of Dr. Jason Birnholz.As you would expect, computer "perception" of digitized ultrasound images should have very different sensitivities than human vision. Sometime in the 1980s, I presented a poster at the American Institute of Ultrasound in Medicine (AIUM) annual meeting of liver ultrasound images that were color-coded by computed standard deviation of signal amplitude in sequential nearest-neighbor regions. There were definite separations of normal and biopsy-proven cirrhotic cases. This work was supported by ultrasound vendor ATL, which chose not to pursue it. There had been a lot of interest in automated pattern recognition at the time in higher-order statistical features of images that could not be seen by direct viewing but which are grist for a computational mill.
A better, current example is the Visualize:Vascular system from ultrasound company Salient Imaging that maps the lumen of arteries precisely from a stack of (noisy) B-mode images. The program unlocks a wealth of valuable, quantitative information that just cannot be seen in the originals, addressing first and foremost precise quantitation of lumen compromise by atherosclerotic plaque.
The ultrasound conundrum
Evidence-based medicine, as it is typically known, has already had a profound effect on practice and on uniform practice delivery. Unfortunately, most large-scale studies involving ultrasound show that the modality does not have much utility, accuracy, or reliability. That this is true after decades of global ultrasound use saddens me. Mixing all forms of ultrasound in mass population analyses can only reflect the dominant form of ultrasound as it is utilized, and that is mainly the limited-use type. This form is inefficient and overreimbursed, and progress will remain impeded so long as this is the default mode.
Limited-use ultrasound does have an important role in care. It is easy to implement, and there are many situations -- such as needle guidance -- where its use really requires no knowledge of ultrasound at all. The problem is that success in this limited arena promotes technical stasis, and this is the very last thing that any medical discipline can afford now. Ultrasound has not reached its potential as a diagnostic tool addressing multiple potential forms of pathology or as a means of achieving tissue and tissue-state equivalent to a biopsy or to a host of invasive physiologic monitoring techniques.
Ultrasound involves very complex interactions between streams of phonons and tissues. Effective use necessitates continued instrument optimization during an exam, albeit somewhat less acutely with the most recent low-noise imaging devices. Ultrasound is different in this regard from all other medical imaging modalities. Many radiology departments have failed to appreciate or blithely ignore this factor. The scope and reliability of their services has fallen off from the grand frontier days of the 1980s. Even something that seems inconsequential, such as frivolous dynamic range compensation, has consequences.
Recognizing the multiple personalities of ultrasound is the first step in rejuvenating the field. There are some major potential changes in ultrasound imaging on the near horizon. I hope that they will be received and put to work enthusiastically and expertly by a new generation of medical imagers with the backgrounds to utilize them effectively and to integrate them with emerging molecular foundations.
Dr. Jason Birnholz was one of the few advanced academic fellows of the James Picker Foundation, and he has been a professor of radiology and obstetrics. He is a fellow of the American College of Radiology and the Royal College of Radiology, and he was an associate fellow of the American College of Obstetricians and Gynecologists.
The comments and observations expressed herein do not necessarily reflect the opinions of AuntMinnie.com.