Tired of waiting for U.S. Food and Drug Administration (FDA) clearance of ultrasound contrast agents for radiology applications? You're not alone. Off-label use of ultrasound contrast could unlock significant clinical benefits now, according to Dr. Michelle Robbin of the University of Alabama at Birmingham (UAB).
With vigilant planning, training, and marketing, contrast-enhanced ultrasound (CEUS) can be performed successfully for off-label applications, Robbin said. She shared her experience with integrating CEUS into her practice during a presentation at the recent American Institute of Ultrasound in Medicine (AIUM) annual meeting in New York City.
Having been involved in 14 phase II/III trials for ultrasound contrast agents since 1995, Robbin said she got tired of waiting for FDA clearance of ultrasound contrast for radiology.
"For a while I wouldn't speak on it at all because it seemed like we were never going to get FDA-approved," Robbin said. "I finally just said, 'I give up; we're just going to use this clinically.' There's plenty of literature from the rest of the world that says this is an absolutely fabulous technique and we should be using it."
A problem solver
The FDA has already approved three ultrasound contrast agents for cardiology use in the U.S. But approval for radiology applications has languished, despite the fact that contrast has been used for years for radiology procedures all over the world.
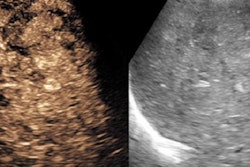
Contrast ultrasound has many advantages: It is a problem-solving tool, offering finer detail than CT/MRI because of the partial-volume effect associated with those modalities, she said.
"We're looking at 2-, 2.5-, 3-mm slices, [with] no radiation, no nephrotoxicity, and a lower cost," Robbin said.
Fortunately, the same contrast agents that have been approved for cardiology can be used off-label for radiology applications -- in fact, they can be used for any clinical application involving blood flow that can be seen with ultrasound, according to Robbin. CEUS is also used as an intracavitary agent, such as in the urinary bladder in pediatric patients and for sonohysterosalpingograms.
Unlike CT or MRI contrast agents, ultrasound contrast agents remain in the vessels; they're not diffused or taken up into the parenchyma.
"The nice part about this is that you can do multiple injections, and then you can do high [mechanical index] flashes so you can break all the bubbles and watch the bubbles wash back in," she said. "And that can all be performed in a single injection with a microbolus technique."
Many areas of CEUS research are currently underway, including evaluating perfusion of tumors, monitoring tumor vascular response to therapy, and targeting chemotherapeutic agents to microbubbles in order to deliver chemotherapy directly to the tumor, she said.
"The sky is the limit," Robbin said.
Before conducting any research with ultrasound contrast, it's important to check with your institutional review board, she said. For example, the institutional review board at UAB requires an FDA investigational new drug (IND) application or exemption from an IND for research involving off-label use of a drug.
Clinical use
If you want to start to use contrast agents off-label for clinical purposes, you have to decide what organs and areas of interest to focus on, Robbin said. Either the kidney or liver is a great place to begin.
"Start with one or two key areas and get another radiologist with you," she said.
While some radiologist colleagues may be reluctant to use drugs off-label, Robbin noted that interventional radiologists and other nonradiology colleagues often employ off-label technology.
"Especially talk to your [interventional radiology] people if you want to get kind of an attitude adjustment, because they use everything off-label," she said.
Another example of routine off-label use in radiology is the ProHance MR contrast agent (Bracco Diagnostics), which is used in thousands of cases each year in the U.S. for evaluating liver lesions.
"Guess what? That's off-label use and nobody knows that," Robbin said.
You'll also need to have two or three radiologists who are comfortable reading CEUS studies, as well as two or three sonographers who are comfortable performing the examination. Typically, in the U.S., the sonographer performs the study.
CEUS also comes with some technical considerations, including the need for an advanced high-end ultrasound scanner with an installed contrast package.
"You need to test it before you can have a patient," she said. "Sometimes [the vendor does] an upgrade and everything -- all your presets -- will be gone."
You'll also need to make sure your institution's PACS can handle the three-minute video clips from CEUS studies. In the past, that hasn't always been the case.
"The very first time you do that, you should have an application specialist [there] from the company," she said. "And don't accept an application specialist who doesn't have firsthand experience with the machine. Otherwise, you'll lose your bolus, and depending on how much [contrast agent] you give [the patient], that might be your only bolus."
Workflow issues
Do contrast ultrasound studies require informed consent from patients? It depends on your hospital; Robbin recommends mirroring your practice in CT or MRI.
At UAB, a general interview sheet for the patient is completed and then signed by the nurse or doctor just prior to study. The nurse calculates the maximum dose of contrast based on the patient's weight and records it on the interview sheet.
Institutions also need to determine who will start the IV, as well as who will prepare and inject the contrast.
"We actually have a nurse start the IV and prepare and inject the contrast," she said. "We do this as a procedure."
Noting that CT and MR technologists start IVs and administer contrast, Robbin said that "at some point it would be nice if we had sonographers who do that."
Patients are also scheduled for CEUS studies in advance for Monday through Friday, between 8 a.m. and 5 p.m.
"When we call the patient, we call it a 'bubble study' because we were freaking patients out," Robbin said. "We would call and say, we've got your contrast study scheduled, and [sometimes] they would say, 'Oh no no no. My doctor told me never to get contrast.' So now we call it a bubble study."
A focused CEUS exam can take 30 to 45 minutes of room time, including the patient interview and IV. Robbin pointed out that workflow can also be improved if radiologists review prior imaging ahead of time. This allows the IV to be started prior to the ultrasound.
She noted that it's important to perform good-quality grayscale imaging first and make measurements prior to initiating the CEUS study.
"It's nice to have normal parenchyma for comparison; don't just look at that area," she said. "And a lesion needs to be within about 10 cm [from the skin surface]. It's best if you can see the lesion in quiet respiration."
The actual CEUS exam rarely takes more than five to 10 minutes, most of which is spent getting a good image of the lesion, Robbin said.
Marketing
In reporting on lesions that are indeterminate on CT, for example, Robbin uses a standard speech recognition macro that includes language recommending CEUS for further evaluation of the patient.
It's also important to develop radiology internal consensus about indications, so that uniform recommendations can be made for CEUS. Market this service to your colleagues, she advised.
"[Radiologists] need to recommend contrast ultrasound and stop recommending CT for incidental renal lesions, or at least the indeterminate ones," she said.
Contrast ultrasound should be used for renal and liver lesions in the following patients:
- Those with renal insufficiency
- Those with end-stage renal disease who still make urine
- Those with a CT or MRI contrast allergy
- Those with renal lesions that are indeterminate on CT or MRI
- Young patients (to avoid ionizing radiation)
Radiologists also need to market CEUS to their referring physicians. This could include, for example, presenting at clinical conferences and giving grand rounds.
"Also, follow up your cases and pull your colleagues into the reading room [to look at the cases with you]," Robbin said. "The urologists are going to love it, the nephrologists like it, and our transplant surgeons like it a lot."
As of today, the FDA has cleared three ultrasound contrast agents for cardiac indications: Definity (Lantheus Medical Imaging), Optison (GE Healthcare), and Lumason (Bracco). These agents can also be used off-label in radiology.
"I would use whatever your cardiology department uses," she said. "Then, if you run out, you can borrow it, and if they run out, they can borrow it."
These agents still have an FDA black box warning for the possibility of serious cardiopulmonary reactions. Most of these serious reactions occur within 30 minutes of injection. While the incidence of these reactions is extremely rare, CEUS should be performed with resuscitation equipment and trained personnel available.
Does patient blood pressure or oxygenation need to be monitored during or after a routine CEUS study? Robbin recommends mirroring your echocardiography practice.
"UAB cardiology doesn't, so we don't," she said.