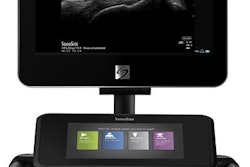
Fujifilm VisualSonics has received clearance from the U.S. Food and Drug Administration (FDA) for its Vevo MD ultrahigh-frequency clinical ultrasound system.
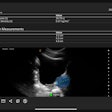
The device is the first foray from Fujifilm VisualSonics into the clinical market. It produces images with resolution down to 30 micrometers and is compatible with the firm's high-frequency series of transducers, which can operate in a range of frequencies up to 70 MHz, the company said.
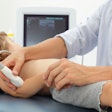
Vevo MD is designed for clinical applications such as neonatology, dermatology, and vascular and musculoskeletal imaging, according to the firm.
Fujifilm VisualSonics is a subsidiary of ultrasound developer Fujifilm SonoSite.