While vital for high-quality care, accreditation can be challenging and sometimes tedious for imaging departments. To help, this article series will offer some tips for how to comply with the latest Intersocietal Accreditation Commission (IAC) Quality Improvement (QI) requirements with quality and ease.
Part 1 focused on the first two required QI measures: test appropriateness and technical quality. This article will address the other two key QI measures for all imaging modalities to follow: interpretive quality and report completeness.
 Judith Buckland, president of CardioServ.
Judith Buckland, president of CardioServ.Interpretive quality
The third QI measure -- interpretive quality -- requires that all modalities now evaluate the quality and accuracy of the interpretation based on the acquired images. Again, this was already part of the QI program for most of the imaging modalities under other names. For echocardiography there was little change; the previous physician peer review/variability program met this standard. On June 8, the IAC released the new guidelines for both adult and pediatric echocardiography with an effective date of January 1, 2017. The new standards removed the requirements to assess specific pathology. Other modalities -- nuclear medicine/PET, CT, and MRI -- had previous QI measures that included some aspect of interpretive quality assessment as an option under broader categories.
The biggest change was once again seen within the vascular testing division as yet another new measure was added. Although vascular had the most measures added to the program, it also received the greatest reduction in regard to one of the biggest challenges that vascular labs face. No spoiler alerts -- I will cover that in an upcoming article.
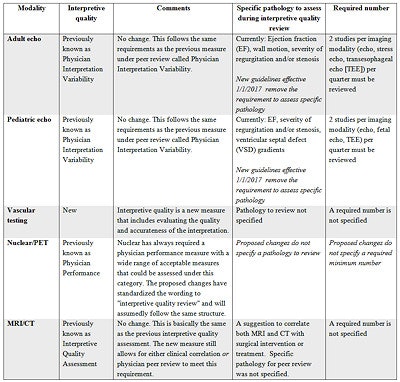
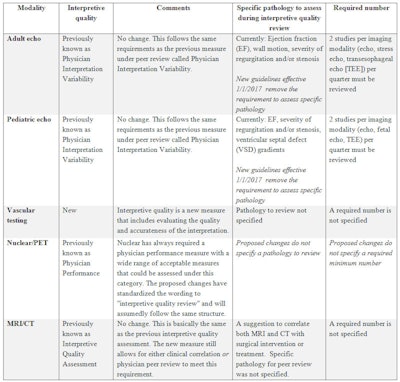
Summary of interpretive quality measure.
Report completeness
The final standardized QI measure implemented in all imaging divisions was report completeness, which requires evaluation of final reports for completeness and timeliness. This requirement is not new to echocardiography and the set requirement of 10 studies per quarter has been reduced to just two per quarter in the latest echo standards with an effective date of January 1, 2017. For all other imaging modalities this is a new requirement without a specified number of reports to evaluate. Another great added benefit to the newly released adult and pediatric QI requirements is the allowance of the same cases to be used to complete the test appropriateness, technical quality, and interpretive quality measures.
Interestingly, although tracking report completeness is new to most imaging divisions, the IAC has always had clear standards on report completeness and timeliness. If a facility did not follow these reporting standards, it risked being issued a "Delayed Status" instead of being granted accreditation. The IAC requires the submission of complete and timely reports to rectify this kind of delayed status.
As an accreditation consultant I have always assessed the completeness of reports as a tool to prepare my clients for successful accreditation. I like this new measure because I feel it helps labs be better prepared for the early identification of report deficiencies. Tips and techniques for completing this measure with ease will be shared in future articles in this series, along with solutions to address reporting deficiencies.
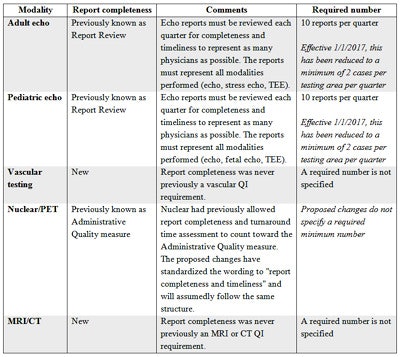
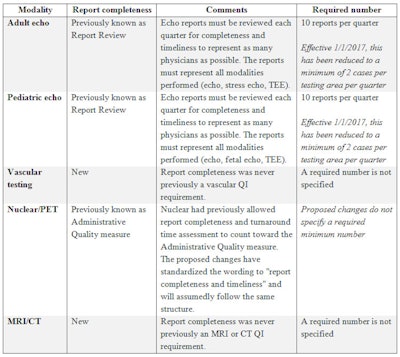
Summary of report completeness measure.
Wild card
We now have reviewed the four mandated across-the-board QI measures for all imaging modalities:
- Test appropriateness
- Technical quality
- Interpretive quality
- Report completeness
As mentioned earlier, though, the IAC is allowing each imaging modality division to include one additional measure, which I like to call the "wild card." We'll cover these in our next article.
Judith Buckland is president of CardioServ, a consulting firm focused on inspiring excellence in diagnostic imaging. Judith can be reached by email at [email protected] or via CardioServ's website.
The comments and observations expressed do not necessarily reflect the opinions of AuntMinnie.com.