Philips Healthcare has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Lumify's S4-1 cardiac ultrasound transducer.
Lumify S4-1 brings new capabilities to the Lumify platform, which launched in the U.S. at the end of 2015. These include cardiac imaging, and the transducer can be marketed for use in ambulatory care and by home health professionals, the company said.
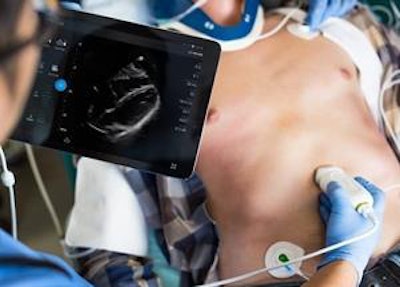
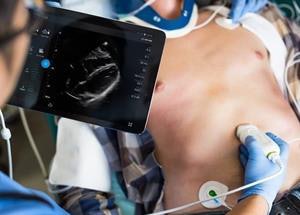 The Lumify S4-1 cardiac ultrasound transducer. Image courtesy of Philips Healthcare.
The Lumify S4-1 cardiac ultrasound transducer. Image courtesy of Philips Healthcare.The smart device is pocket-sized and lightweight, offering advanced sensitivity and very high-resolution 2D image quality, as well as new exam presets, enabling clinicians to triage and assess patients quickly, Philips said. Expanded clinical applications in the latest Lumify model include abdominal, lung, and ob/gyn applications, as well as cardiac, the company said.
All three Lumify transducers (L12-4, C5-2, and S4-1) underwent rigorous environmental and durability testing to ensure its reliability for emergency, critical care, and ambulance use, the company said. The S4-1 transducer and cable are smaller than a smartphone, weighing in at 152 grams, enhancing the versatility and mobility of the device.
Data from the probe is accessible on the cloud-based Philips Healthcare digital platform, the company said.