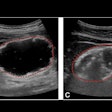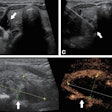
Ultrasound technology start-up Clarius Mobile Health has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Clarius C3 and Clarius L7 app-based ultrasound scanners.
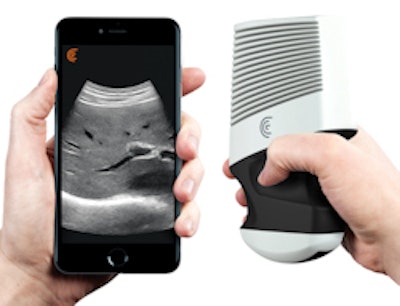
 The Clarius ultrasound device.
The Clarius ultrasound device.
Clarius C3 is a multipurpose ultrasound scanner that can image the abdomen and lungs; it also incorporates a virtual phased array for quick scans of the heart. Clarius L7 is designed to help clinicians guide procedures and image superficial structures.