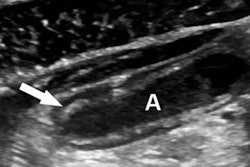
The U.S. Food and Drug Administration (FDA) has approved a label update for Lantheus Medical Imaging's Definity ultrasound contrast agent.
The update removes from the label's prescribing information section a contraindication statement related to use of the agent in patients with a known or suspected cardiac shunt. These patients were previously excluded from receiving echocardiography contrast studies of the left ventricle, according to the firm.
The FDA's decision to remove the contraindication statement was based on a Lantheus regulatory submission that referenced publications and data that support the safety of echocardiography contrast products in patients with cardiac shunts, the company said.