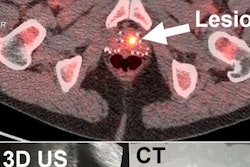
Ultrasound probe manufacturer Interson has received U.S. Food and Drug Administration (FDA) 510(k) clearance for the use of its Simpli series of linear and convex array ultrasound probes in human applications.
The lightweight probes, which can be connected to Windows OS tablets, laptops, and desktops with the USB probe cable, are suitable for point-of-care applications such as primary care, long-term care, rehabilitation, telemedicine, and women's health, according to the company. The Simpli series has been available for veterinary applications since January.