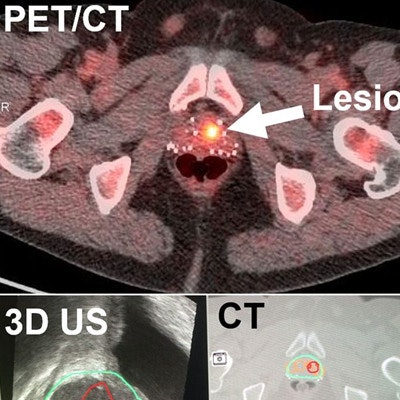
DENVER - Researchers at Emory University are reporting early success in fusing fluciclovine-PET and 3D ultrasound images to better detect and biopsy suspected recurrent prostate cancer. They presented their findings at the Society of Nuclear Medicine and Molecular Imaging (SNMMI) meeting.
Results from the early-stage clinical trial show that the fusion-targeted biopsy imaging technique increased positive biopsy results and might become a useful method to detect recurrent prostate cancer, especially in patients with prior negative biopsy.
 Baowei Fei, PhD, from Emory University.
Baowei Fei, PhD, from Emory University."By combining the two imaging modalities, we have improved the detection rate for prostate cancer," said lead author Baowei Fei, PhD, an associate professor of radiology and imaging sciences. "We are very excited about the results. We are looking forward to having an immediate impact on the management of recurrent prostate cancer patients."
Need for new options
According to the U.S. National Cancer Institute (NCI), there are an estimated 161,000 new cases of prostate cancer each year in the U.S., along with approximately 26,700 deaths from the disease. The current standard for diagnosis is transrectal ultrasound (TRUS)-guided biopsy, which uses 2D ultrasound images.
"Unfortunately, the current standard of care, TRUS-guided biopsy, can leave undetected as much as 30% of prostate cancer," Fei said. "There are also many patients who have rising prostate-specific antigen (PSA) levels but negative results. That does not mean these patients do not have prostate cancer, because the current technology can miss the cancer."
Supported by funds from the U.S. National Institutes of Health (NIH)/NCI, the Emory researchers were able to begin a clinical trial to explore the novel concept of fusing PET and 3D ultrasound for prostate cancer.
"PET imaging is much more sensitive [in detecting cancer] than ultrasound, because PET provides metabolic information," Fei explained. "And using 3D ultrasound instead of 2D ultrasound, we can better detect the location of the suspicious lesion."
Patient cohort
Fei and colleagues enrolled 27 patients with rising PSA levels after nonprostatectomy local therapy who underwent a dual time-point PET/CT scan (Discovery 690, GE Healthcare) after injection of a fluciclovine radiopharmaceutical (347.8 ± 81.4 MBq). A 3D ultrasound scan was also performed several days before biopsy.
Fluciclovine was developed at Emory University School of Medicine and is marketed under the brand name Axumin by Blue Earth Diagnostics. The PET radiotracer received U.S. Food and Drug Administration (FDA) approval in May 2016 for use in PET studies to identify suspected sites of prostate cancer recurrence in men who have elevated PSA levels after treatment.
In the current study, the researchers identified and measured suspicious lesions based on maximum standardized uptake values (SUVmax), rating the abnormalities as "3" for an equivocal result, "4" for medium concern, or "5" for a highly suspicious lesion.
The noted suspicious regions on PET/CT were delineated and fused to prebiopsy 3D ultrasound images. Another 3D ultrasound scan was then performed during the biopsy session, with those images registered with the PET/CT results for biopsy planning. The PET/ultrasound fusion software is not yet commercially available; Fei is collaborating with a company to make it available for other sites.
As for the imaging fusion process, Fei described it as a "challenging task," given that patients are imaged differently during PET and ultrasound exams.
"PET imaging has the patient lying down on the table, but for the ultrasound, the patient is lying on his side. Therefore, the prostate is deformed," he told AuntMinnie.com. "You have to use a deformable registration method to combine the two images together. Accuracy is the key, because the prostate can deform or move depending on the patient's position."
Imaging results
The researchers detected a total of 62 suspicious lesions among the 27 patents through the fluciclovine-PET/CT scans. The mean SUVmax was 5.4 (± 2.0) at the early time point (4 to 15.5 minutes) for all of the target lesions.
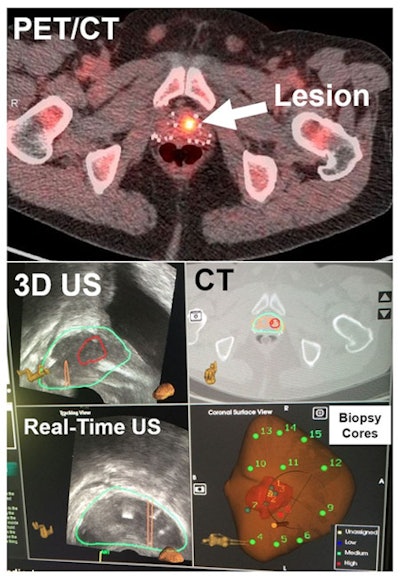 PET/CT (top) shows the lesion in the prostate, followed by CT registered with 3D ultrasound images (middle). Real-time ultrasound images show that the needle during biopsy (bottom left), along with standard 12 cores (cores 4-15) (bottom right), missed the lesion, but PET/ultrasound fusion-targeted biopsy (cores 1-3) detected the prostate cancer. Image courtesy of Baowei Fei, PhD.
PET/CT (top) shows the lesion in the prostate, followed by CT registered with 3D ultrasound images (middle). Real-time ultrasound images show that the needle during biopsy (bottom left), along with standard 12 cores (cores 4-15) (bottom right), missed the lesion, but PET/ultrasound fusion-targeted biopsy (cores 1-3) detected the prostate cancer. Image courtesy of Baowei Fei, PhD.The positivity rate for PET/ultrasound fusion-targeted biopsy increased based on the suspicion level of the lesions, the group found.
| Positivity rate for PET/ultrasound biopsy | |||
| Lesion suspicion level | SUVmax | Cores detected | Positivity rate |
| All lesions | 21 of 62 | 34% | |
| Level 4 and 5 | 5.52 ± 2.1 | 19 of 52 | 36% |
| Level 5 | 6.2 ± 2.1 | 16 of 35 | 48% |
"The positive detection rate using the conventional method of 12-core biopsy is only approximately 7% as reported in the literature," Fei said. "This technology has improved the average positive detection rate to 34%, which is almost five times better."
For the biopsy-verified cancers, the SUVmax was 4.9 (± 2.2) for low-risk prostate cancer and 6.6 (± 2.1) for intermediate- to high-risk prostate cancer.
Based on the improved prostate cancer detection rates, Fei and colleagues concluded that the fusion of fluciclovine-PET and 3D ultrasound images and targeted biopsy technology is helpful for histologic verification of recurrent prostate cancer in this group of patients. This approach should also be studied in the primary disease setting for patients with prior negative biopsy or those on active surveillance, they added.