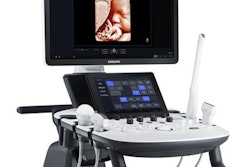
Samsung NeuroLogica has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its RS85A ultrasound scanner.
RS85A includes several key features:
- MV-Flow enables clinicians to see slow-flowing and tiny vascularized structures while examining lesions.
- S-Shearwave Imaging allows clinicians to quantify the elasticity of tissue or lesions to aid in the diagnosis of breast and liver diseases.
- S-Fusion can help guide biopsy and localize lesions through a combination of real-time ultrasound and MRI or CT.
- CEUS+ involves the use of ultrasound contrast to enhance medical images.
The ultrasound scanner is also equipped with an enhanced monitor arm that offers a wide range of motion and tilt to minimize repetitive motions and decrease fatigue, according to the company.
Samsung NeuroLogica will launch RS85A, along with the rest of its ultrasound portfolio, at the American Institute of Ultrasound in Medicine (AIUM) 2018 meeting in New York City.