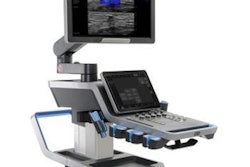
Ultrasound technology developer Exact Imaging has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its FusionVu application on the ExactVu microultrasound system.
FusionVu allows urologists to perform cognitive fusion via the company's Cognitive Assist or microultrasound/MR fusion on ExactVu.
FusionVu also recently received the CE Mark, Exact Imaging said.