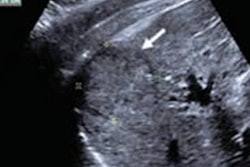
Researchers have developed contrast-enhanced ultrasound (CEUS) software that can accurately predict which patients with a common form of liver cancer will respond to transcatheter arterial chemoembolization (TACE), according to the findings of a June 16 study in Ultrasound in Medicine and Biology.
The custom software enhances the visualization of liver tumor blood vessels for patients with hepatocellular carcinoma (HCC), one of the most prominent types of cancer. By quantifying a tumor's blood vessel features, the researchers were able to identify which patients had tumors that would likely respond to TACE as opposed to another form of treatment.
"The complexity of the tumor vascular network may affect arterial delivery of the drug-eluting beads during TACE treatment," wrote the authors, led by Ipek Oezdemir, a bioengineering graduate student at the University of Texas at Dallas Ultrasound Imaging and Therapy Laboratory. "Thus, quantification of the tumor angiogenic network may provide crucial information to physicians during treatment planning."
Image processing, data extraction, and modeling
During TACE treatment, a catheter is used to deliver chemotherapeutic agents into a tumor's angiogenic network. When successful, TACE completely blocks the tumor blood vessels, but up to 75% of tumors have residual blood flow, requiring repeat treatment or another form of therapy.
Patient response to TACE therapy is typically monitored 4-6 weeks after treatment using contrast-enhanced MRI or contrast-enhanced CT. But the authors hypothesized contrast-enhanced ultrasound may be the better modality -- not only to monitor patients' outcomes but also to visualize tumor vascular networks before treatment.
They tested their hypothesis using imaging and patient data from 36 people with HCC who were participating in a clinical trial evaluating the effectiveness of 2D- and 4D-CEUS for TACE. For the trial, an experienced sonographer performed CEUS scans on patients at three different time points before and after their treatment.
The authors developed a custom MATLAB script to process the CEUS images for better vessel visualization. They only applied the image processing software to the patients' first CEUS scan, which occurred before the start of TACE treatment.
From the processed images, the authors identified six unique features: vessel-to-tissue ratio, number of bifurcations, number of vessels, mean vessel diameter, mean vessel length, and vessel tortuosity. They created models that used the morphological features to predict TACE treatment response and compared the models' predictions with actual patient outcomes.
Up to 86% accuracy
Out of the 33 patients, 19 patients completely responded to TACE, while the remaining had residual blood flow. Patients with an incomplete TACE response had a larger number of vessels and bifurcations on their initial CEUS scan, the authors observed.
Data analysis revealed vessel diameter had the weakest ability to predict TACE treatment response, whereas vessel-to-tissue ratio, number of vessels, and number of bifurcations had the strongest prediction abilities.
The model that used all six morphological features only had 52% accuracy, but the accuracy rose to 72% for the model without the weakest predictor (vessel diameter). When the researchers only included the strongest predictors (vessel-to-tissue ratio, number of bifurcations, and number of vessels), the accuracy jumped to 86%, with a sensitivity of 89% and specificity of 82%.
"Overall these performance metrics illustrated that our model was able to make reliable pretherapeutic HCC responses to TACE predictions," the authors wrote.
Potential for personalized cancer care
The authors hypothesized that vessel features could accurately predict TACE response because more complex vascular networks affect drug delivery. Complex vascular networks can also be a sign of a more aggressive tumor and may reflect tumors that respond better to transarterial radioembolization (TARE) than TACE.
More studies with a larger number of participants are needed to confirm the efficacy of the image processing software and prediction models. But if validated, CEUS imaging may become an invaluable tool to help personalize cancer treatment for patients with HCC.
"A novel CEUS image processing and analysis method was developed that both extracts the morphologic features from tumor vascular network and predicts HCC response to TACE treatment," the authors concluded. "Introduction of a reliable method for predicting TACE response may help provide more effective therapeutic planning and more personalized patient strategies."