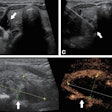
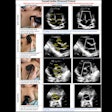
Optoacoustic imaging firm Seno Medical Instruments has received supplemental premarket approval (PMA) from the U.S. Food and Drug Administration (FDA) for its Imagio breast imaging system.
The latest version of Imagio features integration of ultrasound technology into the optoacoustic probe, a new ultrasound probe, and elimination of redundant electronics, according to the vendor. Using a combination of ultrasound and optoacoustic technology, Imagio is designed to aid physicians in differentiating malignant and benign breast masses in real time, Seno said.