Optoacoustic imaging firm Seno Medical Instruments has garnered U.S. Food and Drug Administration (FDA) premarket approval (PMA) for its Imagio breast imaging optoacoustic ultrasound system.
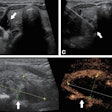
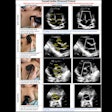
Designed to help providers characterize and differentiate breast masses in real-time, Imagio combines laser optics and grayscale ultrasound to provide fused functional and anatomical breast imaging, according to the vendor. These optoacoustic images provide a blood map in and around breast masses, while the ultrasound technology yields a traditional anatomic image, Seno said. The company believes that Imagio could help to reduce the number of unnecessary diagnostic breast biopsies.
Imagio also comes with SenoGram, an artificial intelligence (AI)-based decision-support tool for aiding radiologists in interpreting the new images and helping them in transitioning from ultrasound alone to optoacoustic ultrasound imaging, the firm said.