An ensemble AI model combining clinical variables, O-RADS, and deep learning radiomics can help in diagnosing ovarian tumors, suggest findings published March 5 in Translational Oncology.
Researchers led by Yimin Wu from East China Normal University in Wuhu, China, found that their model achieved high accuracy and helped improve the performance of sonographers by between 7.7% and 11%.
“The ensemble model significantly enhances preoperative ovarian cancer diagnosis accuracy and improves sonographers' diagnostic capabilities and consistency,” Wu and colleagues wrote.
Radiology researchers continue to explore the potential of deep learning radiomics in more accurately diagnosing diseases through tumor differentiation. In the case of ovarian cancer, transvaginal ultrasound (TVUS) is the first-line modality used for diagnosis, with O-RADS aiding with risk stratification.
The researchers noted that few studies have investigated the potential of a holistic model that integrates clinical factors, O-RADS scores, and deep-learning radiomics to stratify ovarian cancer risk.
Wu and colleagues added to the literature by developing its own ensemble model that includes the aforementioned factors. It also explored the model improved the performance of sonographers.
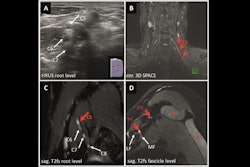
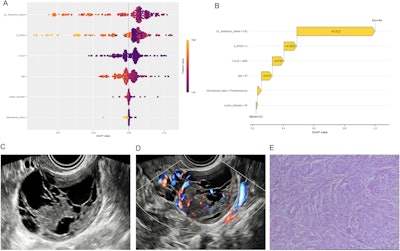 (A) SHAP summary plot shows the global contributions of variables to the model predictions. (B-E) Local explanation of the model for a single instance of ovarian endometrioid carcinoma: SHAP waterfall plot (B), ultrasound grayscale image (C), ultrasound color Doppler image (D), and 10×10 histopathological image (E). Images are available for republishing under a Creative Commons license (CC BY-NC-ND 4.0).
(A) SHAP summary plot shows the global contributions of variables to the model predictions. (B-E) Local explanation of the model for a single instance of ovarian endometrioid carcinoma: SHAP waterfall plot (B), ultrasound grayscale image (C), ultrasound color Doppler image (D), and 10×10 histopathological image (E). Images are available for republishing under a Creative Commons license (CC BY-NC-ND 4.0).
The researchers developed their model by using data from two medical centers, one for training and internal validation and the other for external validation. The training set included 413 patients while the internal validation set included 177 patients. The external validation set meanwhile included 312 patients. The model incorporated 687 radiomics features.
The deep-learning radiomics model achieved high marks in all three sets, based on the least absolute shrinkage and selection operator (LASSO) method.
| Performance of an AI model in training, internal validation, and external validation patient sets | |||
|---|---|---|---|
| Measure | Training set | Internal validation | External validation |
| Area under the curve (AUC) | 0.93 | 0.91 | 0.93 |
| Sensitivity | 87% | 78% | 86% |
| Specificity | 84% | 93% | 87% |
The ensemble model, meanwhile, which combined deep learning radiomics features with clinical characteristics and O-RADS assessment by sonographers achieved an AUC of 0.97 in both internal and external validation groups.
The average AUC values of the sonographers improved by 11% in the internal validation set and by 7.7 % in the external validation set.
The study authors highlighted that their model “offers a more efficient, cost-effective, and interpretable diagnosis of ovarian cancer.”
“While our findings suggest the potential of AI to enhance diagnostic proficiency, tailored training interventions, such as short orientation sessions, structured tutorials, or user-friendly interfaces, could further assist junior sonographers in fully leveraging the model's capabilities,” they wrote.
The team called for future studies to explore the feasibility, effectiveness, and cost-efficiency of these strategies in diverse clinical settings.
The full study can be found here.