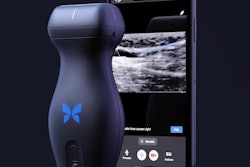
The U.S. Food and Drug Administration (FDA) has cleared Butterfly Network's updated version of its semiconductor-based, handheld point-of-care ultrasound (POCUS) system, Butterfly iQ3.
The updated device features a new ergonomic design and increased speed for image resolution, sensitivity, and penetration, as well as faster 3D capabilities to power new automated image capture modes the company calls iQ Slice and iQ Fan, the company said.